Graft-derived neurons and bystander effects are maintained for six months after human iPSC-derived NESC transplantation in mice's cerebella
- PMID: 38332227
- PMCID: PMC10853537
- DOI: 10.1038/s41598-024-53542-x
Graft-derived neurons and bystander effects are maintained for six months after human iPSC-derived NESC transplantation in mice's cerebella
Abstract
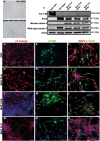
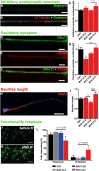
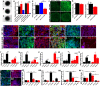
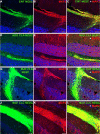
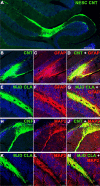
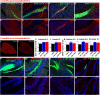
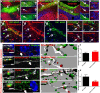
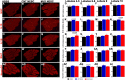
Machado-Joseph disease (MJD) is a neurodegenerative disorder characterized by widespread neuronal death affecting the cerebellum. Cell therapy can trigger neuronal replacement and neuroprotection through bystander effects providing a therapeutic option for neurodegenerative diseases. Here, human control (CNT) and MJD iPSC-derived neuroepithelial stem cells (NESC) were established and tested for their therapeutic potential. Cells' neuroectodermal phenotype was demonstrated. Brain organoids obtained from the Control NESC showed higher mRNA levels of genes related to stem cells' bystander effects, such as BDNF, NEUROD1, and NOTCH1, as compared with organoids produced from MJD NESC, suggesting that Control NESC have a higher therapeutic potential. Graft-derived glia and neurons, such as cells positive for markers of cerebellar neurons, were detected six months after NESC transplantation in mice cerebella. The graft-derived neurons established excitatory and inhibitory synapses in the host cerebella, although CNT neurons exhibited higher excitatory synapse numbers compared with MJD neurons. Cell grafts, mainly CNT NESC, sustained the bystander effects through modulation of inflammatory interleukins (IL1B and IL10), neurotrophic factors (NGF), and neurogenesis-related proteins (Msi1 and NeuroD1), for six months in the mice cerebella. Altogether this study demonstrates the long-lasting therapeutic potential of human iPSC-derived NESC in the cerebellum.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Establishment and characterization of human pluripotent stem cells-derived brain organoids to model cerebellar diseases.Sci Rep. 2022 Jul 22;12(1):12513. doi: 10.1038/s41598-022-16369-y. Sci Rep. 2022. PMID: 35869235 Free PMC article.
-
Ibuprofen enhances synaptic function and neural progenitors proliferation markers and improves neuropathology and motor coordination in Machado-Joseph disease models.Hum Mol Genet. 2019 Nov 15;28(22):3691-3703. doi: 10.1093/hmg/ddz097. Hum Mol Genet. 2019. PMID: 31127937
-
Compromised mitochondrial complex II in models of Machado-Joseph disease.Biochim Biophys Acta. 2012 Feb;1822(2):139-49. doi: 10.1016/j.bbadis.2011.10.010. Epub 2011 Oct 20. Biochim Biophys Acta. 2012. PMID: 22037589 Free PMC article.
-
Reverse engineering human neurodegenerative disease using pluripotent stem cell technology.Brain Res. 2016 May 1;1638(Pt A):30-41. doi: 10.1016/j.brainres.2015.09.023. Epub 2015 Sep 28. Brain Res. 2016. PMID: 26423934 Free PMC article. Review.
-
Stem cell models of human synapse development and degeneration.Mol Biol Cell. 2018 Nov 26;29(24):2913-2921. doi: 10.1091/mbc.E18-04-0222. Mol Biol Cell. 2018. PMID: 30475098 Free PMC article. Review.
References
-
- Mendonca, L. S., Nobrega, C., Hirai, H., Kaspar, B. K. & Pereira de Almeida, L. Transplantation of cerebellar neural stem cells improves motor coordination and neuropathology in Machado-Joseph disease mice. Brain a journal of neurology138, 320–335, doi:10.1093/brain/awu352 (2015). - PubMed
MeSH terms
Grants and funding
- COMPETE 2020 - Operational Programme for Competitiveness and Internationalization/European Regional Development Fund
- COMPETE 2020 - Operational Programme for Competitiveness and Internationalization/European Regional Development Fund
- projects - UIDB/04539/2020 and UIDP/04539/2020, POCI-01-0145-FEDER-030737 (NeuroStemForMJD, PTDC/BTM-ORG/30737/2017), CEECIND/04242/2017, PhD Scholarships 2020.04751.BD and 2020.07385.BD/FCT - Fundação para a Ciência e a Tecnologia
- projects - UIDB/04539/2020 and UIDP/04539/2020, POCI-01-0145-FEDER-030737 (NeuroStemForMJD, PTDC/BTM-ORG/30737/2017), CEECIND/04242/2017, PhD Scholarships 2020.04751.BD and 2020.07385.BD/FCT - Fundação para a Ciência e a Tecnologia
- Trampoline Grant #20126/AFM-Téléthon
LinkOut - more resources
Full Text Sources

