Myosteatosis: Diagnosis, pathophysiology and consequences in metabolic dysfunction-associated steatotic liver disease
- PMID: 38322420
- PMCID: PMC10844870
- DOI: 10.1016/j.jhepr.2023.100963
Myosteatosis: Diagnosis, pathophysiology and consequences in metabolic dysfunction-associated steatotic liver disease
Abstract
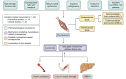
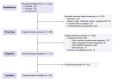
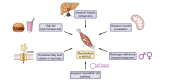
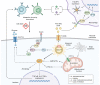
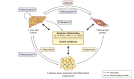
Metabolic dysfunction-associated steatotic liver disease (MASLD) is associated with an increased risk of multisystemic complications, including muscle changes such as sarcopenia and myosteatosis that can reciprocally affect liver function. We conducted a systematic review to highlight innovative assessment tools, pathophysiological mechanisms and metabolic consequences related to myosteatosis in MASLD, based on original articles screened from PUBMED, EMBASE and COCHRANE databases. Forty-six original manuscripts (14 pre-clinical and 32 clinical studies) were included. Microscopy (8/14) and tissue lipid extraction (8/14) are the two main assessment techniques used to measure muscle lipid content in pre-clinical studies. In clinical studies, imaging is the most used assessment tool and included CT (14/32), MRI (12/32) and ultrasound (4/32). Assessed muscles varied across studies but mainly included paravertebral (4/14 in pre-clinical; 13/32 in clinical studies) and lower limb muscles (10/14 in preclinical; 13/32 in clinical studies). Myosteatosis is already highly prevalent in non-cirrhotic stages of MASLD and correlates with disease activity when using muscle density assessed by CT. Numerous pathophysiological mechanisms were found and included: high-fat and high-fructose diet, dysregulation in fatty acid transport and ketogenesis, endocrine disorders and impaired microRNA122 pathway signalling. In this review we also uncover several potential consequences of myosteatosis in MASLD, such as insulin resistance, MASLD progression from steatosis to metabolic steatohepatitis and loss of muscle strength. In conclusion, data on myosteatosis in MASLD are already available. Screening for myosteatosis could be highly relevant in the context of MASLD, considering its correlation with MASLD activity as well as its related consequences.
Keywords: MASH; MASLD; adipokines; hepatokines; inflammation; insulin resistance; liver; metabolic dysfunction-associated steatohepatitis; metabolic dysfunction-associated steatotic liver disease; muscle; myokines; myosteatosis.
© 2023 The Author(s).
Conflict of interest statement
GH received a travel grant from Gilead Sciences. IAL has patents planned, issued, or pending (PCT/EP2022/065769 Ref WO/2022/258788); received research grants from FNRS, Région wallonne and Televie. NL received speaker fees from Gilead Sciences and Fresenius Kabi; received travel grants from Abbvie, Gilead Sciences and Norgine and receives grants from Gilead Sciences. AL has no conflict of interest to declare. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures
Similar articles
-
Impact of Peroxisome Proliferator-Activated Receptor Agonists on Myosteatosis in the Context of Metabolic Dysfunction-Associated Steatotic Liver Disease.Discov Med. 2024 Jun;36(185):1139-1153. doi: 10.24976/Discov.Med.202436185.104. Discov Med. 2024. PMID: 38926100 Review.
-
Unlocking liver health: Can tackling myosteatosis spark remission in metabolic dysfunction-associated steatotic liver disease?Liver Int. 2024 Aug;44(8):1781-1796. doi: 10.1111/liv.15938. Epub 2024 Apr 16. Liver Int. 2024. PMID: 38623714 Review.
-
Systemic impacts of metabolic dysfunction-associated steatotic liver disease (MASLD) and metabolic dysfunction-associated steatohepatitis (MASH) on heart, muscle, and kidney related diseases.Front Cell Dev Biol. 2024 Jul 16;12:1433857. doi: 10.3389/fcell.2024.1433857. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39086662 Free PMC article. Review.
-
Effects of ipragliflozin on skeletal muscle adiposity in patients with diabetes and metabolic dysfunction-associated steatotic liver disease.Intern Med. 2024 Nov 1. doi: 10.2169/internalmedicine.4456-24. Online ahead of print. Intern Med. 2024. PMID: 39496446
-
Association between metabolic dysfunction-associated steatotic liver disease and myosteatosis measured by computed tomography.J Cachexia Sarcopenia Muscle. 2024 Oct;15(5):1942-1952. doi: 10.1002/jcsm.13543. Epub 2024 Jul 16. J Cachexia Sarcopenia Muscle. 2024. PMID: 39011807 Free PMC article.
Cited by
-
Myosteatosis and the clinical outcomes of patients with liver cirrhosis: A meta-analysis.PLoS One. 2024 Sep 12;19(9):e0310017. doi: 10.1371/journal.pone.0310017. eCollection 2024. PLoS One. 2024. PMID: 39264966 Free PMC article.
-
Impact of Physical Activity on Overall Survival and Liver Cirrhosis Incidence in Steatotic Liver Disease: Insights from a Large Cohort Study Using Inverse Probability of Treatment Weighting.Nutrients. 2024 Aug 2;16(15):2532. doi: 10.3390/nu16152532. Nutrients. 2024. PMID: 39125411 Free PMC article.
-
Exploring Varied Treatment Strategies for Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD).Life (Basel). 2024 Jul 3;14(7):844. doi: 10.3390/life14070844. Life (Basel). 2024. PMID: 39063598 Free PMC article. Review.
-
Skeletal muscle size and quality in healthy kidney donors, normal range and clinical associations.Sci Rep. 2024 Oct 24;14(1):25257. doi: 10.1038/s41598-024-76188-1. Sci Rep. 2024. PMID: 39448639 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

