Targeting PERK-ATF4-P21 axis enhances the sensitivity of osteosarcoma HOS cells to Mppα-PDT
- PMID: 38319715
- PMCID: PMC10911341
- DOI: 10.18632/aging.205511
Targeting PERK-ATF4-P21 axis enhances the sensitivity of osteosarcoma HOS cells to Mppα-PDT
Abstract
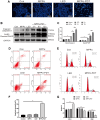
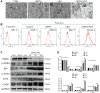
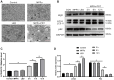
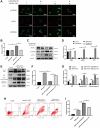
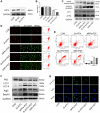
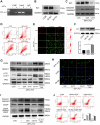
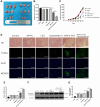
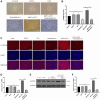
Osteosarcoma (OS) is the most prevalent type of malignant bone tumor in adolescents. The overall survival of OS patients has reached a plateau recently. Thus, there is an urgent need to develop approaches to improve the sensitivity of OS to therapies. Pyropheophorbide-α methyl ester-mediated photodynamic therapy (MPPα-PDT) is a new type of tumor therapy, and elucidating its mechanism is helpful to improve its anti-tumor efficacy. Here, we investigated how PERK signaling promotes the human OS (HOS) cell survival induced by MPPα-PDT, as overcoming this may enhance sensitivity to MPPα-PDT. We found that MPPα-PDT combined with PERK inhibitor GSK2656157 enhanced HOS cell apoptosis by suppressing autophagy and p21. Autophagy inhibition and p21 depletion enhanced cell death, indicating pro-survival effects in MPPα-PDT. Notably, p21 was found to be an effector of the PERK-Atf4 pathway, which could positively regulate autophagy mediated by MPPα-PDT. In conclusion, we found that the combination of MPPα-PDT and GSK2656157 enhanced apoptosis in HOS cells by inhibiting autophagy. Mechanistically, this autophagy is p21-dependent and can be suppressed by GSK2656157, thereby enhancing sensitivity to MPPα-PDT.
Keywords: MPPα-PDT; PERK pathway; apoptosis; autophagy; human osteosarcoma; p21.
Conflict of interest statement
Figures
Similar articles
-
Targeting X box-binding protein-1 (XBP1) enhances the sensitivity of HOS osteosarcoma cells to pyropheophorbide- α methyl ester-mediated photodynamic therapy.Photodiagnosis Photodyn Ther. 2022 Mar;37:102646. doi: 10.1016/j.pdpdt.2021.102646. Epub 2021 Nov 21. Photodiagnosis Photodyn Ther. 2022. PMID: 34818599
-
Targeting GRP78 enhances the sensitivity of HOS osteosarcoma cells to pyropheophorbide-α methyl ester-mediated photodynamic therapy via the Wnt/β-catenin signaling pathway.Acta Biochim Biophys Sin (Shanghai). 2021 Oct 12;53(10):1387-1397. doi: 10.1093/abbs/gmab115. Acta Biochim Biophys Sin (Shanghai). 2021. PMID: 34494093 Free PMC article.
-
Effect and mechanisms of celastrol on the apoptosis of HOS osteosarcoma cells.Oncol Rep. 2018 Oct;40(4):2260-2268. doi: 10.3892/or.2018.6619. Epub 2018 Aug 1. Oncol Rep. 2018. PMID: 30106429
-
The safety and efficiency of photodynamic therapy for the treatment of osteosarcoma: A systematic review of in vitro experiment and animal model reports.Photodiagnosis Photodyn Ther. 2022 Dec;40:103093. doi: 10.1016/j.pdpdt.2022.103093. Epub 2022 Aug 27. Photodiagnosis Photodyn Ther. 2022. PMID: 36031143 Review.
-
Cell apoptosis, autophagy and necroptosis in osteosarcoma treatment.Oncotarget. 2016 Jul 12;7(28):44763-44778. doi: 10.18632/oncotarget.8206. Oncotarget. 2016. PMID: 27007056 Free PMC article. Review.
References
-
- Bielack SS, Kempf-Bielack B, Branscheid D, Carrle D, Friedel G, Helmke K, Kevric M, Jundt G, Kühne T, Maas R, Schwarz R, Zoubek A, Jürgens H. Second and subsequent recurrences of osteosarcoma: presentation, treatment, and outcomes of 249 consecutive cooperative osteosarcoma study group patients. J Clin Oncol. 2009; 27:557–65. 10.1200/JCO.2008.16.2305 - DOI - PubMed
-
- Smeland S, Bielack SS, Whelan J, Bernstein M, Hogendoorn P, Krailo MD, Gorlick R, Janeway KA, Ingleby FC, Anninga J, Antal I, Arndt C, Brown KLB, et al.. Survival and prognosis with osteosarcoma: outcomes in more than 2000 patients in the EURAMOS-1 (European and American Osteosarcoma Study) cohort. Eur J Cancer. 2019; 109:36–50. 10.1016/j.ejca.2018.11.027 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

