Microglial ferroptotic stress causes non-cell autonomous neuronal death
- PMID: 38317225
- PMCID: PMC10840184
- DOI: 10.1186/s13024-023-00691-8
Microglial ferroptotic stress causes non-cell autonomous neuronal death
Abstract
Background: Ferroptosis is a form of regulated cell death characterised by lipid peroxidation as the terminal endpoint and a requirement for iron. Although it protects against cancer and infection, ferroptosis is also implicated in causing neuronal death in degenerative diseases of the central nervous system (CNS). The precise role for ferroptosis in causing neuronal death is yet to be fully resolved.
Methods: To elucidate the role of ferroptosis in neuronal death we utilised co-culture and conditioned medium transfer experiments involving microglia, astrocytes and neurones. We ratified clinical significance of our cell culture findings via assessment of human CNS tissue from cases of the fatal, paralysing neurodegenerative condition of amyotrophic lateral sclerosis (ALS). We utilised the SOD1G37R mouse model of ALS and a CNS-permeant ferroptosis inhibitor to verify pharmacological significance in vivo.
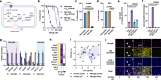
Results: We found that sublethal ferroptotic stress selectively affecting microglia triggers an inflammatory cascade that results in non-cell autonomous neuronal death. Central to this cascade is the conversion of astrocytes to a neurotoxic state. We show that spinal cord tissue from human cases of ALS exhibits a signature of ferroptosis that encompasses atomic, molecular and biochemical features. Further, we show the molecular correlation between ferroptosis and neurotoxic astrocytes evident in human ALS-affected spinal cord is recapitulated in the SOD1G37R mouse model where treatment with a CNS-permeant ferroptosis inhibitor, CuII(atsm), ameliorated these markers and was neuroprotective.
Conclusions: By showing that microglia responding to sublethal ferroptotic stress culminates in non-cell autonomous neuronal death, our results implicate microglial ferroptotic stress as a rectifiable cause of neuronal death in neurodegenerative disease. As ferroptosis is currently primarily regarded as an intrinsic cell death phenomenon, these results introduce an entirely new pathophysiological role for ferroptosis in disease.
Keywords: Amyotrophic lateral sclerosis (ALS); Drug discovery; Ferroptosis; Glia; Glial activation; Iron; Microglia; Neurodegeneration; Neurotoxic astrocytes; Therapy.
© 2024. The Author(s).
Conflict of interest statement
Collaborative Medicinal Development LLC has licensed intellectual property related to this subject from the University of Melbourne where the inventors include ARW and PSD. AIB is a shareholder in Alterity Ltd, Cogstate Ltd, Brighton Biotech LLC, Grunbiotics Pty Ltd, Eucalyptus Pty Ltd, and Mesoblast Ltd. He is a paid consultant for Collaborative Medicinal Development LLC and has a profit share interest in Collaborative Medicinal Development Pty Ltd. PJC and JSB are unpaid consultants for Collaborative Medicinal Development LLC.
Figures




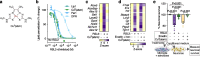


Similar articles
-
Ferroptosis mediates selective motor neuron death in amyotrophic lateral sclerosis.Cell Death Differ. 2022 Jun;29(6):1187-1198. doi: 10.1038/s41418-021-00910-z. Epub 2021 Dec 2. Cell Death Differ. 2022. PMID: 34857917 Free PMC article.
-
The accumulation of enzymatically inactive cuproenzymes is a CNS-specific phenomenon of the SOD1G37R mouse model of ALS and can be restored by overexpressing the human copper transporter hCTR1.Exp Neurol. 2018 Sep;307:118-128. doi: 10.1016/j.expneurol.2018.06.006. Epub 2018 Jun 12. Exp Neurol. 2018. PMID: 29906423
-
Microglia RAGE exacerbates the progression of neurodegeneration within the SOD1G93A murine model of amyotrophic lateral sclerosis in a sex-dependent manner.J Neuroinflammation. 2021 Jun 15;18(1):139. doi: 10.1186/s12974-021-02191-2. J Neuroinflammation. 2021. PMID: 34130712 Free PMC article.
-
Significance of aberrant glial cell phenotypes in pathophysiology of amyotrophic lateral sclerosis.Neurosci Lett. 2017 Jan 1;636:27-31. doi: 10.1016/j.neulet.2016.07.052. Epub 2016 Jul 26. Neurosci Lett. 2017. PMID: 27473942 Review.
-
Little Helpers or Mean Rogue-Role of Microglia in Animal Models of Amyotrophic Lateral Sclerosis.Int J Mol Sci. 2021 Jan 20;22(3):993. doi: 10.3390/ijms22030993. Int J Mol Sci. 2021. PMID: 33498186 Free PMC article. Review.
Cited by
-
The Role of Ferroptosis in Amyotrophic Lateral Sclerosis Treatment.Neurochem Res. 2024 Oct;49(10):2653-2667. doi: 10.1007/s11064-024-04194-w. Epub 2024 Jun 12. Neurochem Res. 2024. PMID: 38864944 Review.
-
Microglial iron trafficking: new player in brain injury.Turk J Med Sci. 2022 Oct;52(5):1415-1424. doi: 10.55730/1300-0144.5481. Epub 2022 Oct 19. Turk J Med Sci. 2022. PMID: 36422479 Free PMC article. Review.
-
Integrated elemental analysis supports targeting copper perturbations as a therapeutic strategy in multiple sclerosis.Neurotherapeutics. 2024 Sep;21(5):e00432. doi: 10.1016/j.neurot.2024.e00432. Epub 2024 Aug 19. Neurotherapeutics. 2024. PMID: 39164165 Free PMC article.
References
-
- Khakh BS, Deneen B. The emerging nature of astrocyte diversity. Annu Rev Neurosci. 2019;42:187–207. - PubMed
-
- Salter MW, Stevens B. Microglia emerge as central players in brain disease. Nat Med. 2017;23(9):1018–1027. - PubMed
-
- Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, et al. Amyotrophic lateral sclerosis. Lancet. 2011;377(9769):942–955. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

