Acute gastrointestinal permeability after traumatic brain injury in mice precedes a bloom in Akkermansia muciniphila supported by intestinal hypoxia
- PMID: 38316862
- PMCID: PMC10844296
- DOI: 10.1038/s41598-024-53430-4
Acute gastrointestinal permeability after traumatic brain injury in mice precedes a bloom in Akkermansia muciniphila supported by intestinal hypoxia
Abstract
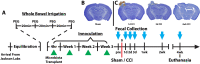
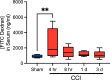
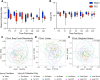
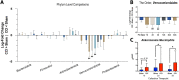
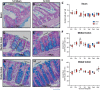
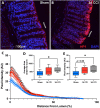
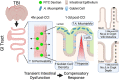
Traumatic brain injury (TBI) increases gastrointestinal morbidity and associated mortality. Clinical and preclinical studies implicate gut dysbiosis as a consequence of TBI and an amplifier of brain damage. However, little is known about the association of gut dysbiosis with structural and functional changes of the gastrointestinal tract after an isolated TBI. To assess gastrointestinal dysfunction, mice received a controlled cortical impact or sham brain injury and intestinal permeability was assessed at 4 h, 8 h, 1 d, and 3 d after injury by oral administration of 4 kDa FITC Dextran prior to euthanasia. Quantification of serum fluorescence revealed an acute, short-lived increase in permeability 4 h after TBI. Despite transient intestinal dysfunction, no overt morphological changes were evident in the ileum or colon across timepoints from 4 h to 4 wks post-injury. To elucidate the timeline of microbiome changes after TBI, 16 s gene sequencing was performed on DNA extracted from fecal samples collected prior to and over the first month after TBI. Differential abundance analysis revealed that the phylum Verrucomicrobiota was increased at 1, 2, and 3 d after TBI. The Verrucomicrobiota species was identified by qPCR as Akkermansia muciniphila, an obligate anaerobe that resides in the intestinal mucus bilayer and produces short chain fatty acids (e.g. butyrate) utilized by intestinal epithelial cells. We postulated that TBI promotes intestinal changes favorable for the bloom of A. muciniphila. Consistent with this premise, the relative area of mucus-producing goblet cells in the medial colon was significantly increased at 1 d after injury, while colon hypoxia was significantly increased at 3 d. Our findings reveal acute gastrointestinal functional changes coupled with an increase of beneficial bacteria suggesting a potential compensatory response to systemic stress after TBI.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Akkermansia muciniphila-Nlrp3 is involved in the neuroprotection of phosphoglycerate mutase 5 deficiency in traumatic brain injury mice.Front Immunol. 2023 May 23;14:1172710. doi: 10.3389/fimmu.2023.1172710. eCollection 2023. Front Immunol. 2023. PMID: 37287985 Free PMC article.
-
Traumatic brain injury and intestinal dysfunction: uncovering the neuro-enteric axis.J Neurotrauma. 2009 Aug;26(8):1353-9. doi: 10.1089/neu.2008.0858. J Neurotrauma. 2009. PMID: 19344293 Free PMC article.
-
Traumatic Brain Injury in Mice Induces Acute Bacterial Dysbiosis Within the Fecal Microbiome.Front Immunol. 2018 Nov 27;9:2757. doi: 10.3389/fimmu.2018.02757. eCollection 2018. Front Immunol. 2018. PMID: 30546361 Free PMC article.
-
Akkermansia muciniphila: key player in metabolic and gastrointestinal disorders.Eur Rev Med Pharmacol Sci. 2019 Sep;23(18):8075-8083. doi: 10.26355/eurrev_201909_19024. Eur Rev Med Pharmacol Sci. 2019. PMID: 31599433 Review.
-
Physiological benefits of Akkermansia muciniphila under high-altitude hypoxia.Appl Microbiol Biotechnol. 2023 Jan;107(1):1-8. doi: 10.1007/s00253-022-12305-2. Epub 2022 Nov 28. Appl Microbiol Biotechnol. 2023. PMID: 36437378 Review.
Cited by
-
Fecal microbiota transplantation alleviates cognitive impairment by improving gut microbiome composition and barrier function in male rats of traumatic brain injury following gas explosion.Front Microbiol. 2024 Nov 1;15:1485936. doi: 10.3389/fmicb.2024.1485936. eCollection 2024. Front Microbiol. 2024. PMID: 39552646 Free PMC article.
-
Probiotics in Traumatic Brain Injury: New Insights into Mechanisms and Future Perspectives.J Clin Med. 2024 Aug 3;13(15):4546. doi: 10.3390/jcm13154546. J Clin Med. 2024. PMID: 39124812 Free PMC article. Review.
-
Akkermansia muciniphila: biology, microbial ecology, host interactions and therapeutic potential.Nat Rev Microbiol. 2024 Oct 15. doi: 10.1038/s41579-024-01106-1. Online ahead of print. Nat Rev Microbiol. 2024. PMID: 39406893 Review.
References
-
- Kemp CD, Cotton B, Johnson C, Weaver K. How we die: The impact of non-neurological organ dysfunction following traumatic brain injury. J. Am. Coll. Surg. 2006;203:S36–S36. doi: 10.1016/j.jamcollsurg.2006.05.090. - DOI
-
- Krishnamoorthy V, Temkin N, Barber J, Foreman B, Komisarow J, Korley FK, Laskowitz DT, Mathew JP, Hernandez A, Sampson J, et al. Association of early multiple organ dysfunction with clinical and functional outcomes over the year following traumatic brain injury: A transforming research and clinical knowledge in traumatic brain injury study. Crit. Care Med. 2021;49:1769–1778. doi: 10.1097/ccm.0000000000005055. - DOI - PMC - PubMed
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

