Crosstalk Between Microbiota, Microbial Metabolites, and Interferons in the Inflammatory Bowel Disease Gut
- PMID: 38314170
- PMCID: PMC10836980
- DOI: 10.1093/jcag/gwad044
Crosstalk Between Microbiota, Microbial Metabolites, and Interferons in the Inflammatory Bowel Disease Gut
Abstract
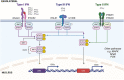
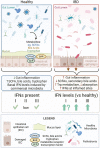
With the prevalence of inflammatory bowel diseases (IBD) continuing to rise in Canada and globally, developing improved therapeutics that successfully treat greater percentages of patients with reduced complications is paramount. A better understanding of pertinent immune pathways in IBD will improve our ability to both successfully dampen inflammation and promote gut healing, beyond just inhibiting specific immune proteins; success of combination therapies supports this approach. Interferons (IFNs) are key cytokines that protect mucosal barrier surfaces, and their roles in regulating gut homeostasis and inflammation differ between the three IFN families (type I, II, and III). Interestingly, the gut microbiota and microbial metabolites impact IFN-signaling, yet how this system is impacted in IBD remains unclear. In this review, we discuss the current knowledge of how gut microbiota directly or indirectly impact IFN levels/responses, and what is known about IFNs differentially regulating gut homeostasis and inflammation in animal models or patients with IBD.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Canadian Association of Gastroenterology.
Figures
Similar articles
-
The interplay between the microbiota, diet and T regulatory cells in the preservation of the gut barrier in inflammatory bowel disease.Front Microbiol. 2023 Dec 1;14:1291724. doi: 10.3389/fmicb.2023.1291724. eCollection 2023. Front Microbiol. 2023. PMID: 38107848 Free PMC article. Review.
-
Impact of Bacterial Metabolites on Gut Barrier Function and Host Immunity: A Focus on Bacterial Metabolism and Its Relevance for Intestinal Inflammation.Front Immunol. 2021 May 26;12:658354. doi: 10.3389/fimmu.2021.658354. eCollection 2021. Front Immunol. 2021. PMID: 34122415 Free PMC article. Review.
-
The Roles of Inflammation, Nutrient Availability and the Commensal Microbiota in Enteric Pathogen Infection.Microbiol Spectr. 2015 Jun;3(3). doi: 10.1128/microbiolspec.MBP-0008-2014. Microbiol Spectr. 2015. PMID: 26185088
-
Type I and Type III Interferons Display Different Dependency on Mitogen-Activated Protein Kinases to Mount an Antiviral State in the Human Gut.Front Immunol. 2017 Apr 21;8:459. doi: 10.3389/fimmu.2017.00459. eCollection 2017. Front Immunol. 2017. PMID: 28484457 Free PMC article.
-
Current insights on the roles of gut microbiota in inflammatory bowel disease-associated extra-intestinal manifestations: pathophysiology and therapeutic targets.Gut Microbes. 2023 Dec;15(2):2265028. doi: 10.1080/19490976.2023.2265028. Epub 2023 Oct 11. Gut Microbes. 2023. PMID: 37822139 Free PMC article. Review.
Cited by
-
Therapeutic potential of a novel pyrazolyl-pyridine derivative in the treatment of experimental colitis.Future Med Chem. 2024;16(19):1971-1982. doi: 10.1080/17568919.2024.2385298. Epub 2024 Aug 19. Future Med Chem. 2024. PMID: 39157857
-
The effect of multiple sclerosis therapy on gut microbiota dysbiosis: a longitudinal prospective study.Microb Cell. 2024 Apr 4;11:106-115. doi: 10.15698/mic2024.03.819. eCollection 2024. Microb Cell. 2024. PMID: 38638559 Free PMC article.
References
-
- Windsor, Joseph W., Kuenzig M. Ellen, Murthy Sanjay K., Bitton Alain, Bernstein Charles N., Jones Jennifer L., Lee Kate,. et al. “The 2023 Impact of Inflammatory Bowel Disease in Canada: Executive Summary.” Journal of the Canadian Association of Gastroenterology 6, no. Supplement_2 (2023): S1–8. 10.1093/jcag/gwad003 - DOI - PMC - PubMed
-
- Kotenko, Sergei V., Gallagher Grant, Baurin Vitaliy V., Lewis-Antes Anita, Shen Meiling, Shah Nital K., Langer Jerome A., Sheikh Faruk, Dickensheets Harold, and Donnelly Raymond P... “IFN-lambdas Mediate Antiviral Protection through a Distinct Class II Cytokine Receptor Complex.” Nature Immunology 4, no. 1 (2003): 69–77. 10.1038/ni875 - DOI - PubMed
-
- Prokunina-Olsson, Ludmila, Muchmore Brian, Tang Wei, Pfeiffer Ruth M., Park Heiyoung, Dickensheets Harold, Hergott Dianna,. et al. “A Variant Upstream of IFNL3 (IL28B) Creating a New Interferon Gene IFNL4 is Associated with Impaired Clearance of Hepatitis C Virus.” Nature Genetics 45, no. 2 (2013): 164–71. 10.1038/ng.2521 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
