Agricultural Waste-Derived Biochar-Based Nitrogenous Fertilizer for Slow-Release Applications
- PMID: 38313543
- PMCID: PMC10831961
- DOI: 10.1021/acsomega.3c06687
Agricultural Waste-Derived Biochar-Based Nitrogenous Fertilizer for Slow-Release Applications
Abstract
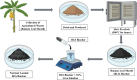
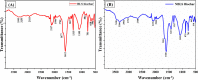
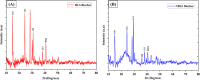
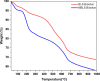
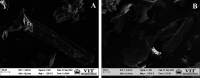
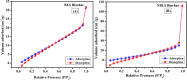
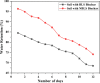
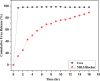
The exponential increase in population demands more food to be produced by employing modern technologies. There is a worldwide increase in the use of chemical fertilizers to rapidly enhance the crop yield. Nitrogen is a crucial plant nutrient, and nitrogenous fertilizers are the most widely used fertilizers. However, the high solubility and volatility of commonly used nitrogenous fertilizers have led to low nutrient use efficiency and alarming environmental pollution. They are lost due to the volatilization of ammonia and leaching of nitrate and release of nitrous oxide, and thus, plants only absorb approximately 20-30% of the nitrogen present in fertilizers. Slow-release fertilizers have been designed to overcome these issues and supply nutrients gradually and sustainably. Biochar, a solid material rich in carbon derived from biomass, can reduce nutrient loss in soil and extend the effectiveness of fertilizers in promoting plant uptake. In the present study, a slow-release nitrogenous fertilizer is prepared using biochar obtained by pyrolysis of a banana leaf sheath (BLS) at 500 °C for 3 h. The BLS biochar and nutrient-loaded BLS (NBLS) biochar exhibited significant water absorbance capacity, water retention capacity, swelling ratio, and equilibrium water content, which would support the maintenance of water levels in soils. The lower salt index values of the prepared fertilizer showed its potential to be used as a sustainable and clean fertilizer. The prepared BLS and NBLS biochar were also characterized by various techniques such as Fourier transform infrared (FT-IR), powder X-ray diffraction, thermogravimetric analysis, scanning electron microscopy, energy-dispersive X-ray spectroscopy, and Brunauer-Emmett-Teller (BET) methods. The FT-IR spectra of both BLS and NBLS biochar demonstrate the existence of primary, secondary, and tertiary alcohols, alkanes, alkenes, esters, and phenols. The peak at 1423 cm-1 in NBLS biochar corresponds to the vibration of NH4+ confirming nutrient loading. A minor phase change was noticed in the intensities of NBLS biochar, which may be attributed to the absorption of nutrients into the structure of biochar. TGA analysis confirmed the stability of BLS and NBLS Biochar. SEM analysis demonstrates a highly porous structure of the biochar samples due to the release of volatile matter from the biomass. The BET-specific surface area of BLS and NBLS biochar was 43.216 and 35.014 m2/g, respectively. Nutrient release studies showed an incremental increase in the nitrogen release percentage over a period of 16 h. The gradual supply of nitrogen to the plants over an extended period demonstrated by the prepared slow-release fertilizer confirms its potential to reduce the leaching loss commonly observed in conventional chemical fertilizers.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
A performance evaluation study of nano-biochar as a potential slow-release nano-fertilizer from wheat straw residue for sustainable agriculture.Chemosphere. 2021 Dec;285:131382. doi: 10.1016/j.chemosphere.2021.131382. Epub 2021 Jul 8. Chemosphere. 2021. PMID: 34329141
-
The preparation of slow-release fertilizers with biomass ash and water/waste acid solutions from desulfurization and denitrification of flue gas.Environ Sci Pollut Res Int. 2022 Aug;29(38):57566-57578. doi: 10.1007/s11356-022-19868-7. Epub 2022 Mar 30. Environ Sci Pollut Res Int. 2022. PMID: 35353314
-
Synthesizing biochar-based fertilizer with sustained phosphorus and potassium release: Co-pyrolysis of nutrient-rich chicken manure and Ca-bentonite.Sci Total Environ. 2022 May 20;822:153509. doi: 10.1016/j.scitotenv.2022.153509. Epub 2022 Jan 29. Sci Total Environ. 2022. PMID: 35101507
-
Controlled release fertilizers (CRFs) for climate-smart agriculture practices: a comprehensive review on release mechanism, materials, methods of preparation, and effect on environmental parameters.Environ Sci Pollut Res Int. 2022 Aug;29(36):53967-53995. doi: 10.1007/s11356-022-20890-y. Epub 2022 May 28. Environ Sci Pollut Res Int. 2022. PMID: 35624378 Review.
-
Nitrate and Nitrogen Oxides: Sources, Health Effects and Their Remediation.Rev Environ Contam Toxicol. 2017;242:183-217. doi: 10.1007/398_2016_11. Rev Environ Contam Toxicol. 2017. PMID: 27734212 Review.
Cited by
-
Impact of Pyrolysis Temperature on the Physical and Chemical Properties of Non-Modified Biochar Produced from Banana Leaves: A Case Study on Ammonium Ion Adsorption.Materials (Basel). 2024 Jun 28;17(13):3180. doi: 10.3390/ma17133180. Materials (Basel). 2024. PMID: 38998263 Free PMC article.
References
-
- FAO . World Fertilizer Trends and Outlook to 2020; Food and Agriculture Organization of the United Nations, 2017.
-
- Fu J.; Wang C.; Chen X.; Huang Z.; Chen D. Classification Research and Types of Slow Controlled Release Fertilizers (SRFs) Used - a Review. Commun. Soil Sci. Plant Anal. 2018, 49 (17), 2219–2230. 10.1080/00103624.2018.1499757. - DOI
-
- Shahzad A. N.; Qureshi M. K.; Wakeel A.; Misselbrook T. Crop Production in Pakistan and Low Nitrogen Use Efficiencies. Nat. Sustain. 2019, 2 (12), 1106–1114. 10.1038/s41893-019-0429-5. - DOI
-
- Torres-Martínez J. A.; Mora A.; Knappett P. S. K.; Ornelas-Soto N.; Mahlknecht J. Tracking Nitrate and Sulfate Sources in Groundwater of an Urbanized Valley Using a Multi-Tracer Approach Combined with a Bayesian Isotope Mixing Model. Water Res. 2020, 182, 11596210.1016/j.watres.2020.115962. - DOI - PubMed
-
- An X.; Yu J.; Yu J.; Tahmasebi A.; Wu Z.; Liu X.; Yu B. Incorporation of Biochar into Semi-Interpenetrating Polymer Networks through Graft Co-Polymerization for the Synthesis of New Slow-Release Fertilizers. J. Clean. Prod. 2020, 272, 12273110.1016/j.jclepro.2020.122731. - DOI
LinkOut - more resources
Full Text Sources
