High pathogenicity avian influenza A (H5N1) clade 2.3.4.4b virus infection in a captive Tibetan black bear (Ursus thibetanus): investigations based on paraffin-embedded tissues, France, 2022
- PMID: 38305177
- PMCID: PMC10913436
- DOI: 10.1128/spectrum.03736-23
High pathogenicity avian influenza A (H5N1) clade 2.3.4.4b virus infection in a captive Tibetan black bear (Ursus thibetanus): investigations based on paraffin-embedded tissues, France, 2022
Abstract
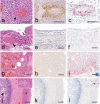
High pathogenicity avian influenza viruses (HPAIVs) H5Nx of clade 2.3.4.4b have been circulating increasingly in both wild and domestic birds in recent years. In turn, this has led to an increase in the number of spillover events affecting mammals. In November 2022, an HPAIV H5N1 caused an outbreak in a zoological park in the south of France, resulting in the death of a Tibetan black bear (Ursus thibetanus) and several captive and wild bird species. We detected the virus in various tissues of the bear and a wild black-headed gull (Chroicocephalus ridibundus) found dead in its enclosure using histopathology, two different in situ detection techniques, and next-generation sequencing, all performed on formalin-fixed paraffin-embedded tissues. Phylogenetic analysis performed on the hemagglutinin gene segment showed that bear and gull strains shared 99.998% genetic identity, making the bird strain the closest related strain. We detected the PB2 E627K mutation in minute quantities in the gull, whereas it predominated in the bear, which suggests that this mammalian adaptation marker was selected during the bear infection. Our results provide the first molecular and histopathological characterization of an H5N1 virus infection in this bear species.
Importance: Avian influenza viruses are able to cross the species barrier between birds and mammals because of their high genetic diversity and mutation rate. Using formalin-fixed paraffin-embedded tissues, we were able to investigate a Tibetan black bear's infection by a high pathogenicity H5N1 avian influenza virus at the molecular, phylogenetic, and histological levels. Our results highlight the importance of virological surveillance programs in mammals and the importance of raising awareness among veterinarians and zookeepers of the clinical presentations associated with H5Nx virus infection in mammals.
Keywords: epidemiology; influenza; zoonotic infections.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Investigating the Genetic Diversity of H5 Avian Influenza Viruses in the United Kingdom from 2020-2022.Microbiol Spectr. 2023 Aug 17;11(4):e0477622. doi: 10.1128/spectrum.04776-22. Epub 2023 Jun 26. Microbiol Spectr. 2023. PMID: 37358418 Free PMC article.
-
Multiple Introductions of Reassorted Highly Pathogenic Avian Influenza H5Nx Viruses Clade 2.3.4.4b Causing Outbreaks in Wild Birds and Poultry in The Netherlands, 2020-2021.Microbiol Spectr. 2022 Apr 27;10(2):e0249921. doi: 10.1128/spectrum.02499-21. Epub 2022 Mar 14. Microbiol Spectr. 2022. PMID: 35286149 Free PMC article.
-
Highly Pathogenic Avian Influenza A(H5Nx) Virus of Clade 2.3.4.4b Emerging in Tibet, China, 2021.Microbiol Spectr. 2022 Jun 29;10(3):e0064322. doi: 10.1128/spectrum.00643-22. Epub 2022 Apr 21. Microbiol Spectr. 2022. PMID: 35446151 Free PMC article.
-
The genetics of highly pathogenic avian influenza viruses of subtype H5 in Germany, 2006-2020.Transbound Emerg Dis. 2021 May;68(3):1136-1150. doi: 10.1111/tbed.13843. Epub 2020 Sep 29. Transbound Emerg Dis. 2021. PMID: 32964686 Review.
-
Iceland as Stepping Stone for Spread of Highly Pathogenic Avian Influenza Virus between Europe and North America.Emerg Infect Dis. 2022 Dec;28(12):2383-2388. doi: 10.3201/eid2812.221086. Epub 2022 Oct 19. Emerg Infect Dis. 2022. PMID: 36261139 Free PMC article. Review.
Cited by
-
Highly Pathogenic Avian Influenza Virus A(H5N1) Clade 2.3.4.4b Infection in Free-Ranging Polar Bear, Alaska, USA.Emerg Infect Dis. 2024 Aug;30(8):1660-1663. doi: 10.3201/eid3008.240481. Epub 2024 Jun 28. Emerg Infect Dis. 2024. PMID: 38941966 Free PMC article.
-
The mammary glands of cows abundantly display receptors for circulating avian H5 viruses.J Virol. 2024 Nov 19;98(11):e0105224. doi: 10.1128/jvi.01052-24. Epub 2024 Oct 10. J Virol. 2024. PMID: 39387556 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

