A macrophage-collagen fragment axis mediates subcutaneous adipose tissue remodeling in mice
- PMID: 38300872
- PMCID: PMC10861897
- DOI: 10.1073/pnas.2313185121
A macrophage-collagen fragment axis mediates subcutaneous adipose tissue remodeling in mice
Abstract
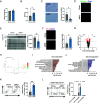
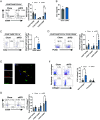
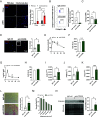
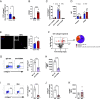
Efficient removal of fibrillar collagen is essential for adaptive subcutaneous adipose tissue (SAT) expansion that protects against ectopic lipid deposition during weight gain. Here, we used mice to further define the mechanism for this collagenolytic process. We show that loss of collagen type-1 (CT1) and increased CT1-fragment levels in expanding SAT are associated with proliferation of resident M2-like macrophages that display increased CD206-mediated engagement in collagen endocytosis compared to chow-fed controls. Blockage of CD206 during acute high-fat diet-induced weight gain leads to SAT CT1-fragment accumulation associated with elevated inflammation and fibrosis markers. Moreover, these SAT macrophages' engagement in collagen endocytosis is diminished in obesity associated with elevated levels collagen fragments that are too short to assemble into triple helices. We show that such short fragments provoke M2-macrophage proliferation and fibroinflammatory changes in fibroblasts. In conclusion, our data delineate the importance of a macrophage-collagen fragment axis in physiological SAT expansion. Therapeutic targeting of this process may be a means to prevent pathological adipose tissue remodeling, which in turn may reduce the risk for obesity-related metabolic disorders.
Keywords: CD206; collagen; fibrosis; macrophages; subcutaneous adipose tissue.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures

Similar articles
-
Pentraxin 3 deficiency protects from the metabolic inflammation associated to diet-induced obesity.Cardiovasc Res. 2019 Nov 1;115(13):1861-1872. doi: 10.1093/cvr/cvz068. Cardiovasc Res. 2019. PMID: 30859179
-
High-fat diet action on adiposity, inflammation, and insulin sensitivity depends on the control low-fat diet.Nutr Res. 2013 Nov;33(11):952-60. doi: 10.1016/j.nutres.2013.07.017. Epub 2013 Sep 14. Nutr Res. 2013. PMID: 24176235
-
Emodin inhibits lipid accumulation and inflammation in adipose tissue of high-fat diet-fed mice by inducing M2 polarization of adipose tissue macrophages.FASEB J. 2021 Jul;35(7):e21730. doi: 10.1096/fj.202100157RR. FASEB J. 2021. PMID: 34110631
-
Molecular mechanism of obesity-induced adipose tissue inflammation; the role of Mincle in adipose tissue fibrosis and ectopic lipid accumulation.Endocr J. 2020 Feb 28;67(2):107-111. doi: 10.1507/endocrj.EJ19-0417. Epub 2019 Dec 19. Endocr J. 2020. PMID: 31852849 Review.
-
What have human experimental overfeeding studies taught us about adipose tissue expansion and susceptibility to obesity and metabolic complications?Int J Obes (Lond). 2017 Jun;41(6):853-865. doi: 10.1038/ijo.2017.4. Epub 2017 Jan 12. Int J Obes (Lond). 2017. PMID: 28077863 Review.
Cited by
-
IL-1β promotes adipogenesis by directly targeting adipocyte precursors.Nat Commun. 2024 Sep 11;15(1):7957. doi: 10.1038/s41467-024-51938-x. Nat Commun. 2024. PMID: 39261467 Free PMC article.
-
Biomaterial-mediated intracellular control of macrophages for cell therapy in pro-inflammatory and pro-fibrotic conditions.Biomaterials. 2024 Jul;308:122545. doi: 10.1016/j.biomaterials.2024.122545. Epub 2024 Mar 22. Biomaterials. 2024. PMID: 38547831 Free PMC article.
References
-
- Schoettl T., Fischer I. P., Ussar S., Heterogeneity of adipose tissue in development and metabolic function. J. Exp. Biol. 221, jeb162958 (2018). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

