Molecular basis and evolutionary drivers of endosperm-based hybridization barriers
- PMID: 38298124
- PMCID: PMC11060687
- DOI: 10.1093/plphys/kiae050
Molecular basis and evolutionary drivers of endosperm-based hybridization barriers
Abstract
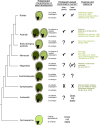
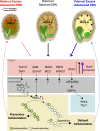
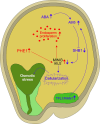
The endosperm, a transient seed tissue, plays a pivotal role in supporting embryo growth and germination. This unique feature sets flowering plants apart from gymnosperms, marking an evolutionary innovation in the world of seed-bearing plants. Nevertheless, the importance of the endosperm extends beyond its role in providing nutrients to the developing embryo by acting as a versatile protector, preventing hybridization events between distinct species and between individuals with different ploidy. This phenomenon centers on growth and differentiation of the endosperm and the speed at which both processes unfold. Emerging studies underscore the important role played by type I MADS-box transcription factors, including the paternally expressed gene PHERES1. These factors, along with downstream signaling pathways involving auxin and abscisic acid, are instrumental in regulating endosperm development and, consequently, the establishment of hybridization barriers. Moreover, mutations in various epigenetic regulators mitigate these barriers, unveiling a complex interplay of pathways involved in their formation. In this review, we discuss the molecular underpinnings of endosperm-based hybridization barriers and their evolutionary drivers.
© The Author(s) 2024. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Conflict of interest statement
Conflict of interest statement. None declared.
Figures
Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
Far Posterior Approach for Rib Fracture Fixation: Surgical Technique and Tips.JBJS Essent Surg Tech. 2024 Dec 6;14(4):e23.00094. doi: 10.2106/JBJS.ST.23.00094. eCollection 2024 Oct-Dec. JBJS Essent Surg Tech. 2024. PMID: 39650795 Free PMC article.
-
Unlocking data: Decision-maker perspectives on cross-sectoral data sharing and linkage as part of a whole-systems approach to public health policy and practice.Public Health Res (Southampt). 2024 Nov 20:1-30. doi: 10.3310/KYTW2173. Online ahead of print. Public Health Res (Southampt). 2024. PMID: 39582242
-
Corticosteroids for the prevention and treatment of bronchopulmonary dysplasia: an overview of systematic reviews.Cochrane Database Syst Rev. 2024 Apr 10;4(4):CD013271. doi: 10.1002/14651858.CD013271.pub2. Cochrane Database Syst Rev. 2024. PMID: 38597338 Review.
Cited by
-
Unveiling the imprinted dance: how parental genomes orchestrate seed development and hybrid success.Front Plant Sci. 2024 Sep 27;15:1455685. doi: 10.3389/fpls.2024.1455685. eCollection 2024. Front Plant Sci. 2024. PMID: 39399543 Free PMC article. Review.
-
Parental dialectic: Epigenetic conversations in endosperm.Curr Opin Plant Biol. 2024 Oct;81:102591. doi: 10.1016/j.pbi.2024.102591. Epub 2024 Jun 29. Curr Opin Plant Biol. 2024. PMID: 38944896 Review.
-
Celebrating the American Society of Plant Biologists centennial anniversary: A compendium of review articles in plant biology.Plant Physiol. 2024 Apr 30;195(1):1-3. doi: 10.1093/plphys/kiae141. Plant Physiol. 2024. PMID: 38547371 Free PMC article. No abstract available.
-
Celebrating the American Society of Plant Biologists centennial anniversary: A compendium of review articles in plant biology.Plant Cell. 2024 May 1;36(5):1183-1185. doi: 10.1093/plcell/koae076. Plant Cell. 2024. PMID: 38466716 Free PMC article. No abstract available.
-
Progress of ABA function in endosperm cellularization and storage product accumulation.Plant Cell Rep. 2024 Nov 20;43(12):287. doi: 10.1007/s00299-024-03378-6. Plant Cell Rep. 2024. PMID: 39565413 Review.
References
-
- Baek YS, Royer SM, Broz AK, Covey PA, López-Casado G, Nuñez R, Kear PJ, Bonierbale M, Orillo M, van der Knaap E, et al. . Interspecific reproductive barriers between sympatric populations of wild tomato species (Solanum section Lycopersicon). Am J Bot. 2016:103(11):1964–1978. 10.3732/ajb.1600356 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

