Phosphorylation of AQP4 by LRRK2 R1441G impairs glymphatic clearance of IFNγ and aggravates dopaminergic neurodegeneration
- PMID: 38296953
- PMCID: PMC10831045
- DOI: 10.1038/s41531-024-00643-z
Phosphorylation of AQP4 by LRRK2 R1441G impairs glymphatic clearance of IFNγ and aggravates dopaminergic neurodegeneration
Abstract
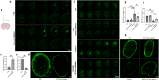
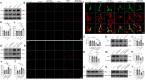
Aquaporin-4 (AQP4) is essential for normal functioning of the brain's glymphatic system. Impaired glymphatic function is associated with neuroinflammation. Recent clinical evidence suggests the involvement of glymphatic dysfunction in LRRK2-associated Parkinson's disease (PD); however, the precise mechanism remains unclear. The pro-inflammatory cytokine interferon (IFN) γ interacts with LRRK2 to induce neuroinflammation. Therefore, we examined the AQP4-dependent glymphatic system's role in IFNγ-mediated neuroinflammation in LRRK2-associated PD. We found that LRRK2 interacts with and phosphorylates AQP4 in vitro and in vivo. AQP4 phosphorylation by LRRK2 R1441G induced AQP4 depolarization and disrupted glymphatic IFNγ clearance. Exogeneous IFNγ significantly increased astrocyte expression of IFNγ receptor, amplified AQP4 depolarization, and exacerbated neuroinflammation in R1441G transgenic mice. Conversely, inhibiting LRRK2 restored AQP4 polarity, improved glymphatic function, and reduced IFNγ-mediated neuroinflammation and dopaminergic neurodegeneration. Our findings establish a link between LRRK2-mediated AQP4 phosphorylation and IFNγ-mediated neuroinflammation in LRRK2-associated PD, guiding the development of LRRK2 targeting therapy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Decreased AQP4 Expression Aggravates ɑ-Synuclein Pathology in Parkinson's Disease Mice, Possibly via Impaired Glymphatic Clearance.J Mol Neurosci. 2021 Dec;71(12):2500-2513. doi: 10.1007/s12031-021-01836-4. Epub 2021 Mar 26. J Mol Neurosci. 2021. PMID: 33772424
-
Dystrophin 71 deficiency causes impaired aquaporin-4 polarization contributing to glymphatic dysfunction and brain edema in cerebral ischemia.Neurobiol Dis. 2024 Sep;199:106586. doi: 10.1016/j.nbd.2024.106586. Epub 2024 Jun 29. Neurobiol Dis. 2024. PMID: 38950712
-
Long-term isoflurane anesthesia induces cognitive deficits via AQP4 depolarization mediated blunted glymphatic inflammatory proteins clearance.J Cereb Blood Flow Metab. 2024 Aug;44(8):1450-1466. doi: 10.1177/0271678X241237073. Epub 2024 Mar 5. J Cereb Blood Flow Metab. 2024. PMID: 38443763 Free PMC article.
-
Astrocytes, HIV and the Glymphatic System: A Disease of Disrupted Waste Management?Front Cell Infect Microbiol. 2020 Sep 29;10:523379. doi: 10.3389/fcimb.2020.523379. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33134185 Free PMC article. Review.
-
The Role of Glymphatic System in Alzheimer's and Parkinson's Disease Pathogenesis.Biomedicines. 2022 Sep 13;10(9):2261. doi: 10.3390/biomedicines10092261. Biomedicines. 2022. PMID: 36140362 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

