Targeted therapeutic hypothermia protects against noise induced hearing loss
- PMID: 38292902
- PMCID: PMC10826421
- DOI: 10.3389/fnins.2023.1296458
Targeted therapeutic hypothermia protects against noise induced hearing loss
Abstract
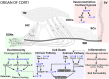
Introduction: Exposure to occupational or recreational loud noise activates multiple biological regulatory circuits and damages the cochlea, causing permanent changes in hearing sensitivity. Currently, no effective clinical therapy is available for the treatment or mitigation of noise-induced hearing loss (NIHL). Here, we describe an application of localized and non-invasive therapeutic hypothermia and targeted temperature management of the inner ear to prevent NIHL.
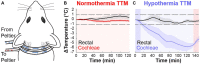
Methods: We developed a custom-designed cooling neck collar to reduce the temperature of the inner ear by 3-4°C post-injury to deliver mild therapeutic hypothermia.
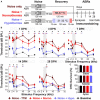
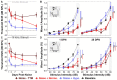
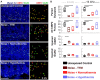
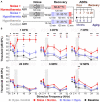
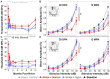
Results: This localized and non-invasive therapeutic hypothermia successfully mitigated NIHL in rats. Our results show that mild hypothermia can be applied quickly and safely to the inner ear following noise exposure. We show that localized hypothermia after NIHL preserves residual hearing and rescues noise-induced synaptopathy over a period of months.
Discussion: This study establishes a minimally-invasive therapeutic paradigm with a high potential for rapid translation to the clinic for long-term preservation of hearing health.
Keywords: hair cells; hidden hearing loss; neuroprotection; noise-induced hearing loss; synaptopathy; therapeutic hypothermia.
Copyright © 2024 Rincon Sabatino, Rivero, Sangaletti, Dietrich, Hoffer, King and Rajgurua.
Conflict of interest statement
SRa and CK are inventors of the intellectual property (IP) used in this study. SRa and the University of Miami may receive royalties for the commercialization of the IP. SRa and CK are co-founders of RestorEar Devices LLC. RestorEar did not provide any financial support for the work described in this manuscript. All conflict of interests for SRa are disclosed to and managed by the University of Miami. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Transcriptional response to mild therapeutic hypothermia in noise-induced cochlear injury.Front Neurosci. 2024 Jan 17;17:1296475. doi: 10.3389/fnins.2023.1296475. eCollection 2023. Front Neurosci. 2024. PMID: 38298897 Free PMC article.
-
Theoretical Evaluation and Experimental Validation of Localized Therapeutic Hypothermia Application to Preserve Residual Hearing After Cochlear Implantation.Ear Hear. 2018 Jul/Aug;39(4):712-719. doi: 10.1097/AUD.0000000000000529. Ear Hear. 2018. PMID: 29240567 Free PMC article.
-
A cool approach to reducing electrode-induced trauma: Localized therapeutic hypothermia conserves residual hearing in cochlear implantation.Hear Res. 2016 Sep;339:32-9. doi: 10.1016/j.heares.2016.05.015. Epub 2016 May 31. Hear Res. 2016. PMID: 27260269 Free PMC article.
-
Cochlear Proteins Associated with Noise-induced Hearing Loss: An Update.Indian J Occup Environ Med. 2018 May-Aug;22(2):60-73. doi: 10.4103/ijoem.IJOEM_43_18. Indian J Occup Environ Med. 2018. PMID: 30319226 Free PMC article. Review.
-
Prevention of Noise-Induced Hearing Loss Using Investigational Medicines for the Inner Ear: Previous Trial Outcomes Should Inform Future Trial Design.Antioxid Redox Signal. 2022 Jun;36(16-18):1171-1202. doi: 10.1089/ars.2021.0166. Epub 2021 Oct 4. Antioxid Redox Signal. 2022. PMID: 34346254 Free PMC article. Review.
Cited by
-
Transcriptional response to mild therapeutic hypothermia in noise-induced cochlear injury.Front Neurosci. 2024 Jan 17;17:1296475. doi: 10.3389/fnins.2023.1296475. eCollection 2023. Front Neurosci. 2024. PMID: 38298897 Free PMC article.
References
-
- Alva N., Carbonell T., Palomeque J. (2004). A model of deep experimental hypothermia and rewarming in rat. J. Therm. Biol. 29, 259–264. doi: 10.1016/j.jtherbio.2004.04.001 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

