SRF-deficient astrocytes provide neuroprotection in mouse models of excitotoxicity and neurodegeneration
- PMID: 38289036
- PMCID: PMC10857791
- DOI: 10.7554/eLife.95577
SRF-deficient astrocytes provide neuroprotection in mouse models of excitotoxicity and neurodegeneration
Erratum in
-
Correction: SRF-deficient astrocytes provide neuroprotection in mouse models of excitotoxicity and neurodegeneration.Elife. 2024 Jul 1;13:e101107. doi: 10.7554/eLife.101107. Elife. 2024. PMID: 38949864 Free PMC article.
Abstract
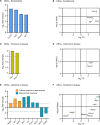
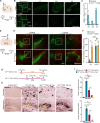
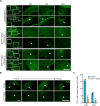
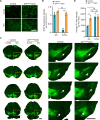
Reactive astrogliosis is a common pathological hallmark of CNS injury, infection, and neurodegeneration, where reactive astrocytes can be protective or detrimental to normal brain functions. Currently, the mechanisms regulating neuroprotective astrocytes and the extent of neuroprotection are poorly understood. Here, we report that conditional deletion of serum response factor (SRF) in adult astrocytes causes reactive-like hypertrophic astrocytes throughout the mouse brain. These SrfGFAP-ERCKO astrocytes do not affect neuron survival, synapse numbers, synaptic plasticity or learning and memory. However, the brains of Srf knockout mice exhibited neuroprotection against kainic-acid induced excitotoxic cell death. Relevant to human neurodegenerative diseases, SrfGFAP-ERCKO astrocytes abrogate nigral dopaminergic neuron death and reduce β-amyloid plaques in mouse models of Parkinson's and Alzheimer's disease, respectively. Taken together, these findings establish SRF as a key molecular switch for the generation of reactive astrocytes with neuroprotective functions that attenuate neuronal injury in the setting of neurodegenerative diseases.
Keywords: SRF; astrocytes; astrogliosis; mouse; neuroprotection; neuroscience; reactive astrocytes; serum response factor.
© 2024, Thumu et al.
Conflict of interest statement
ST, MJ, SS, SD, VV, AN, DG, BJ, DN, JC, SM, NR No competing interests declared
Figures
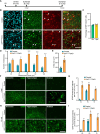
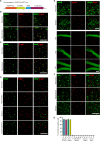
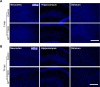
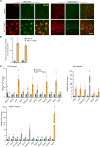
Similar articles
-
SRF Is Required for Maintenance of Astrocytes in Non-Reactive State in the Mammalian Brain.eNeuro. 2021 Jan 29;8(1):ENEURO.0447-19.2020. doi: 10.1523/ENEURO.0447-19.2020. Print 2021 Jan-Feb. eNeuro. 2021. PMID: 33441399 Free PMC article.
-
Astrocyte-Derived Estrogen Regulates Reactive Astrogliosis and is Neuroprotective following Ischemic Brain Injury.J Neurosci. 2020 Dec 9;40(50):9751-9771. doi: 10.1523/JNEUROSCI.0888-20.2020. Epub 2020 Nov 6. J Neurosci. 2020. PMID: 33158962 Free PMC article.
-
Glycolytic preconditioning in astrocytes mitigates trauma-induced neurodegeneration.Elife. 2021 Sep 2;10:e69438. doi: 10.7554/eLife.69438. Elife. 2021. PMID: 34473622 Free PMC article.
-
Astroglial atrophy in Alzheimer's disease.Pflugers Arch. 2019 Oct;471(10):1247-1261. doi: 10.1007/s00424-019-02310-2. Epub 2019 Sep 13. Pflugers Arch. 2019. PMID: 31520182 Review.
-
Therapeutic Strategy of Targeting Astrocytes for Neuroprotection in Parkinson's Disease.Curr Pharm Des. 2017;23(33):4936-4947. doi: 10.2174/1381612823666170710163731. Curr Pharm Des. 2017. PMID: 28699520 Review.
References
-
- Abjean L, Ben Haim L, Riquelme-Perez M, Gipchtein P, Derbois C, Palomares M-A, Petit F, Hérard A-S, Gaillard M-C, Guillermier M, Gaudin-Guérif M, Aurégan G, Sagar N, Héry C, Dufour N, Robil N, Kabani M, Melki R, De la Grange P, Bemelmans AP, Bonvento G, Deleuze J-F, Hantraye P, Flament J, Bonnet E, Brohard S, Olaso R, Brouillet E, Carrillo-de Sauvage M-A, Escartin C. Reactive astrocytes promote proteostasis in Huntington’s disease through the JAK2-STAT3 pathway. Brain. 2023;146:149–166. doi: 10.1093/brain/awac068. - DOI - PubMed
-
- Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, Deik AA, Ginty DD, Clish CB, Gu C. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron. 2017;94:581–594. doi: 10.1016/j.neuron.2017.03.043. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- DST/SJF/LSA-01/2012-2013/Department of Science and Technology, Ministry of Science and Technology, India
- CRG/2019/006899/Science and Engineering Research Board
- BT/PR27952/INF/22/212/2018/Department of Biotechnology, Ministry of Science and Technology, India
- EMR/2015/001946/Science and Engineering Research Board
- DST/INSPIRE/04-I/2016-000002/Department of Science and Technology, Ministry of Science and Technology, India
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

