Selective screening for inborn errors of metabolism using tandem mass spectrometry in West Kazakhstan children: study protocol
- PMID: 38283151
- PMCID: PMC10811460
- DOI: 10.3389/fgene.2023.1278750
Selective screening for inborn errors of metabolism using tandem mass spectrometry in West Kazakhstan children: study protocol
Abstract
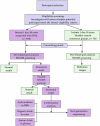
Data on the prevalence of most inborn errors of metabolism are still unavailable in Kazakhstan. The study aims to perform selective screening for hereditary metabolic diseases among patients aged from 1 day to 18 years in western Kazakhstan using the LC-MS/MS method, with establishing the reference values for the content of amino acids, acylcarnitines, and succinylacetone in blood samples of healthy children. Tasks: 1. To assess the burden of metabolic disorders detected by LC-MS/MS in western Kazakhstan by examination of children at clinical risk in pediatric clinics throughout the region; https://www.frontiersin.org/register?returnUrl=https://loop.frontiersin.org 2. To set the reference values of metabolites in the child population; 3. To analyze the age distribution, prevalence, and age of onset for each identified IEM, further comparing the obtained findings with those from previously published reports in other populations.
Methods: To set the reference values of 51 metabolites in the child population, 750 healthy children will be included. The selective screening will be performed among 1,500 patients aged 1 day to 18 years with suspected hereditary metabolic disorders.
Anticipated results: The results of selective screening will be interpreted by comparison with the reference values established. Diagnosis will be based on clinical signs, blood levels of amino acids, acylcarnitines, succinylacetone, and urine levels of organic acids and tests for gene mutations. An assessment of 37 inborn errors of metabolism frequencies in high-risk children will be performed. The research will further develop the national as selective as expanded newborn screening programs. The study was registered in clinicaltrials. gov (https://www.
Clinicaltrials: gov/study/NCT05910151) on 16 June 2023.
Keywords: Kazakhstan; acylcarnitines; amino acids; inborn errors of metabolism; selective screening; succinylacetone; tandem mass spectrometry.
Copyright © 2024 Zharmakhanova, Kononets, Balmagambetova, Syrlybayeva, Nurbaulina, Zhussupova, Sakhanova, Ayaganov, Kim and Zhumalina.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Reference values of amino acids, acylcarnitines and succinylacetone by tandem mass spectrometry for use in newborn screening in southwest Colombia.Colomb Med (Cali). 2017 Sep 30;48(3):113-119. doi: 10.25100/cm.v48i3.2180. Colomb Med (Cali). 2017. PMID: 29213153 Free PMC article.
-
Moroccan Experience of Targeted Screening for Inborn Errors of Metabolism by Tandem Mass Spectrometry.Pediatr Rep. 2023 Mar 10;15(1):227-236. doi: 10.3390/pediatric15010018. Pediatr Rep. 2023. PMID: 36976725 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns.Clin Chem. 2003 Nov;49(11):1797-817. doi: 10.1373/clinchem.2003.022178. Clin Chem. 2003. PMID: 14578311 Review.
-
Clinical effectiveness and cost-effectiveness of neonatal screening for inborn errors of metabolism using tandem mass spectrometry: a systematic review.Health Technol Assess. 2004 Mar;8(12):iii, 1-121. doi: 10.3310/hta8120. Health Technol Assess. 2004. PMID: 14982654 Review.
Cited by
-
Global trends and collaborative networks in gut microbiota-insulin resistance research: a comprehensive bibliometric analysis (2000-2024).Front Med (Lausanne). 2024 Aug 15;11:1452227. doi: 10.3389/fmed.2024.1452227. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39211341 Free PMC article.
References
-
- CLSI (2013). “Blood collection on filter paper for newborn screening programs; approved standard - sixth edition,” in CLSI document NBS01-A6 (Wayne, PA: The Clinical and Laboratory Standards Institute; ).
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources