Cloning, characterization, and spatio-temporal expression patterns of HdhSPARC and its responses to multiple stressors
- PMID: 38278828
- PMCID: PMC10817941
- DOI: 10.1038/s41598-024-51950-7
Cloning, characterization, and spatio-temporal expression patterns of HdhSPARC and its responses to multiple stressors
Abstract
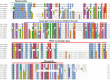
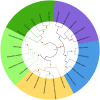
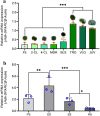
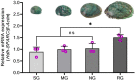
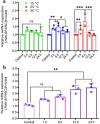
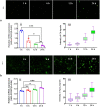
SPARC is an extracellular Ca2+-binding, secreted glycoprotein that plays a dynamic role in the growth and development of organisms. This study aimed to describe the isolation, characterization, and expression analysis of HdhSPARC in Pacific abalone (Haliotis discus hannai) to infer its potential functional role. The isolated HdhSPARC was 1633 bp long, encoding a polypeptide of 284 amino acid residues. Structurally, the SPARC protein in abalone is comprised of three biological domains. However, the structure of this protein varied between vertebrates and invertebrates, as suggested by their distinct clustering patterns in phylogenetic analysis. In early development, HdhSPARC was variably expressed, and higher expression was found in veliger larvae. Moreover, HdhSPARC was highly expressed in juvenile abalone with rapid growth compared to their slower-growing counterparts. Among the testicular development stages, the growth stage exhibited higher HdhSPARC expression. HdhSPARC was also upregulated during muscle remodeling and shell biomineralization, as well as in response to different stressors such as heat shock, LPS, and H2O2 exposure. However, this gene was downregulated in Cd-exposed abalone. The present study first comprehensively characterized the HdhSPARC gene, and its spatio-temporal expressions were analyzed along with its responses to various stressors.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

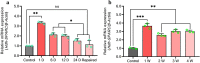
Similar articles
-
EF-Hand-Binding Secreted Protein Hdh-SMP5 Regulates Shell Biomineralization and Responses to Stress in Pacific Abalone, Haliotis discus hannai.Curr Issues Mol Biol. 2023 Dec 13;45(12):10079-10096. doi: 10.3390/cimb45120629. Curr Issues Mol Biol. 2023. PMID: 38132475 Free PMC article.
-
Molecular characterization and expression analysis of a heat shock protein 90 gene from disk abalone (Haliotis discus).Mol Biol Rep. 2011 Jun;38(5):3055-60. doi: 10.1007/s11033-010-9972-x. Epub 2010 Feb 4. Mol Biol Rep. 2011. PMID: 20131011
-
Molecular cloning, characterization and expression analysis of heat shock protein 90 from Pacific abalone, Haliotis discus hannai Ino in response to dietary selenium.Fish Shellfish Immunol. 2011 Jan;30(1):280-6. doi: 10.1016/j.fsi.2010.10.019. Epub 2010 Oct 29. Fish Shellfish Immunol. 2011. PMID: 21044885
-
Structural and functional characterization of Hdh-HSBP1 and its involvement in heat stress and early development in Pacific abalone, Haliotis discus hannai.Fish Shellfish Immunol. 2024 Aug;151:109660. doi: 10.1016/j.fsi.2024.109660. Epub 2024 Jun 1. Fish Shellfish Immunol. 2024. PMID: 38830519
-
Characterization of Insulin-Like Growth Factor Binding Protein 7 (Igfbp7) and Its Potential Involvement in Shell Formation and Metamorphosis of Pacific Abalone, Haliotis discus hannai.Int J Mol Sci. 2020 Sep 7;21(18):6529. doi: 10.3390/ijms21186529. Int J Mol Sci. 2020. PMID: 32906674 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

