Mesenchymal Stem Cell-Based Therapies in the Post-Acute Neurological COVID Syndrome: Current Landscape and Opportunities
- PMID: 38275749
- PMCID: PMC10813738
- DOI: 10.3390/biom14010008
Mesenchymal Stem Cell-Based Therapies in the Post-Acute Neurological COVID Syndrome: Current Landscape and Opportunities
Abstract
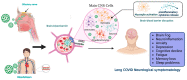
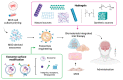
One of the main concerns related to SARS-CoV-2 infection is the symptoms that could be developed by survivors, known as long COVID, a syndrome characterized by persistent symptoms beyond the acute phase of the infection. This syndrome has emerged as a complex and debilitating condition with a diverse range of manifestations affecting multiple organ systems. It is increasingly recognized for affecting the Central Nervous System, in which one of the most prevalent manifestations is cognitive impairment. The search for effective therapeutic interventions has led to growing interest in Mesenchymal Stem Cell (MSC)-based therapies due to their immunomodulatory, anti-inflammatory, and tissue regenerative properties. This review provides a comprehensive analysis of the current understanding and potential applications of MSC-based interventions in the context of post-acute neurological COVID-19 syndrome, exploring the underlying mechanisms by which MSCs exert their effects on neuroinflammation, neuroprotection, and neural tissue repair. Moreover, we discuss the challenges and considerations specific to employing MSC-based therapies, including optimal delivery methods, and functional treatment enhancements.
Keywords: exosomes; long COVID; mesenchymal stem cells; neurological sequelae.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the writing of the manuscript; or in the decision to publish it.
Figures
Similar articles
-
Stalling SARS-CoV2 infection with stem cells: can regenerating perinatal tissue mesenchymal stem cells offer a multi-tiered therapeutic approach to COVID-19?Placenta. 2022 Jan;117:161-168. doi: 10.1016/j.placenta.2021.12.005. Epub 2021 Dec 6. Placenta. 2022. PMID: 34915433 Free PMC article. Review.
-
Mechanisms of Potential Therapeutic Utilization of Mesenchymal Stem Cells in COVID-19 Treatment.Cell Transplant. 2023 Jan-Dec;32:9636897231184611. doi: 10.1177/09636897231184611. Cell Transplant. 2023. PMID: 37395459 Free PMC article. Review.
-
Mesenchymal stromal cells to fight SARS-CoV-2: Taking advantage of a pleiotropic therapy.Cytokine Growth Factor Rev. 2021 Apr;58:114-133. doi: 10.1016/j.cytogfr.2020.12.002. Epub 2020 Dec 15. Cytokine Growth Factor Rev. 2021. PMID: 33397585 Free PMC article. Review.
-
Mesenchymal stem cells and their derived exosomes to combat Covid-19.Rev Med Virol. 2022 Mar;32(2):e2281. doi: 10.1002/rmv.2281. Epub 2021 Aug 7. Rev Med Virol. 2022. PMID: 34363275 Free PMC article. Review.
-
Is there a place for mesenchymal stromal cell-based therapies in the therapeutic armamentarium against COVID-19?Stem Cell Res Ther. 2021 Jul 27;12(1):425. doi: 10.1186/s13287-021-02502-7. Stem Cell Res Ther. 2021. PMID: 34315546 Free PMC article. Review.
Cited by
-
The Role of Dental-derived Stem Cell-based Therapy and Their Derived Extracellular Vesicles in Post-COVID-19 Syndrome-induced Tissue Damage.Stem Cell Rev Rep. 2024 Nov;20(8):2062-2103. doi: 10.1007/s12015-024-10770-y. Epub 2024 Aug 16. Stem Cell Rev Rep. 2024. PMID: 39150646 Review.
References
-
- World Health Organization WHO Coronavirus (COVID-19) Dashboard. [(accessed on 14 November 2023)]. Available online: https://covid19.who.int/
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

