Immunogenicity and protective efficacy of tuzin protein as a vaccine candidate in Leishmania donovani-infected BALB/c mice
- PMID: 38274802
- PMCID: PMC10808571
- DOI: 10.3389/fimmu.2023.1294397
Immunogenicity and protective efficacy of tuzin protein as a vaccine candidate in Leishmania donovani-infected BALB/c mice
Abstract
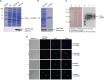
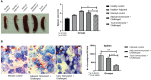
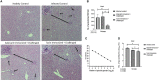
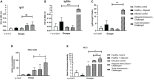
Visceral leishmaniasis (VL) is referred to as the most severe and fatal type of leishmaniasis basically caused by Leishmania donovani and L. infantum. The most effective method for preventing the spread of the disease is vaccination. Till today, there is no promising licensed vaccination for human VL. Hence, investigation for vaccines is necessary to enrich the therapeutic repertoire against leishmaniasis. Tuzin is a rare trans-membrane protein that has been reported in Trypanosoma cruzi with unknown function. However, tuzin is not characterized in Leishmania parasites. In this study, we for the first time demonstrated that tuzin protein was expressed in both stages (promastigote and amastigote) of L. donovani parasites. In-silico studies revealed that tuzin has potent antigenic properties. Therefore, we analyzed the immunogenicity of tuzin protein and immune response in BALB/c mice challenged with the L. donovani parasite. We observed that tuzin-vaccinated mice have significantly reduced parasite burden in the spleen and liver compared with the control. The number of granulomas in the liver was also significantly decreased compared with the control groups. We further measured the IgG2a antibody level, a marker of Th1 immune response in VL, which was significantly higher in the serum of immunized mice when compared with the control. Splenocytes stimulated with soluble Leishmania antigen (SLA) displayed a significant increase in NO and ROS levels compared with the control groups. Tuzin-immunized and parasite-challenged mice exhibit a notable rise in the IFN-γ/IL-10 ratio by significantly suppressing IL-10 expression level, an immunosuppressive cytokine that inhibits leishmanicidal immune function and encourages disease progression. In conclusion, tuzin immunizations substantially increase the protective immune response in L. donovani-challenged mice groups compared with control.
Keywords: Leishmaniasis; Tuzin; antibodies; antigenic; cytokine; immunogenicity; vaccine.
Copyright © 2024 Devender, Sebastian, Maurya, Kumar, Anand, Namdeo and Maurya.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Leishmania genome analysis and high-throughput immunological screening identifies tuzin as a novel vaccine candidate against visceral leishmaniasis.Vaccine. 2014 Jun 24;32(30):3816-22. doi: 10.1016/j.vaccine.2014.04.088. Epub 2014 May 9. Vaccine. 2014. PMID: 24814525
-
Live Attenuated Leishmania donovani Centrin Knock Out Parasites Generate Non-inferior Protective Immune Response in Aged Mice against Visceral Leishmaniasis.PLoS Negl Trop Dis. 2016 Aug 31;10(8):e0004963. doi: 10.1371/journal.pntd.0004963. eCollection 2016 Aug. PLoS Negl Trop Dis. 2016. PMID: 27580076 Free PMC article.
-
115 kDa serine protease confers sustained protection to visceral leishmaniasis caused by Leishmania donovani via IFN-γ induced down-regulation of TNF-α mediated MMP-9 activity.Immunobiology. 2013 Jan;218(1):114-26. doi: 10.1016/j.imbio.2012.02.008. Epub 2012 Feb 16. Immunobiology. 2013. PMID: 22440312
-
Intracellular replication-deficient Leishmania donovani induces long lasting protective immunity against visceral leishmaniasis.J Immunol. 2009 Aug 1;183(3):1813-20. doi: 10.4049/jimmunol.0900276. Epub 2009 Jul 10. J Immunol. 2009. PMID: 19592661
-
Generation of growth arrested Leishmania amastigotes: a tool to develop live attenuated vaccine candidates against visceral leishmaniasis.Vaccine. 2014 Jun 30;32(31):3895-901. doi: 10.1016/j.vaccine.2014.05.009. Epub 2014 May 14. Vaccine. 2014. PMID: 24837513 Review.
References
-
- Webb JR, Campos-Neto A, Ovendale PJ, Martin TI, Stromberg EJ, Badaro R, et al. . Human and murine immune responses to a novel Leishmania major recombinant protein encoded by members of a multicopy gene family. Infect Immun (1998) 66(7):3279–89. doi: 10.1128/IAI.66.7.3279-3289.1998 - DOI - PMC - PubMed
-
- Agallou M, Margaroni M, Karagouni E. Cellular vaccination with bone marrow-derived dendritic cells pulsed with a peptide of Leishmania infantum KMP-11 and CpG oligonucleotides induces protection in a murine model of visceral leishmaniasis. Vaccine (2011) 29(31):5053–64. doi: 10.1016/j.vaccine.2011.04.089 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

