The Development of an Advanced Model for Multilayer Human Skin Reconstruction In Vivo
- PMID: 38268973
- PMCID: PMC10804244
- DOI: 10.21769/BioProtoc.4919
The Development of an Advanced Model for Multilayer Human Skin Reconstruction In Vivo
Abstract
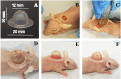
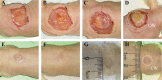
Human skin reconstruction on immune-deficient mice has become indispensable for in vivo studies performed in basic research and translational laboratories. Further advancements in making sustainable, prolonged skin equivalents to study new therapeutic interventions rely on reproducible models utilizing patient-derived cells and natural three-dimensional culture conditions mimicking the structure of living skin. Here, we present a novel step-by-step protocol for grafting human skin cells onto immunocompromised mice that requires low starting cell numbers, which is essential when primary patient cells are limited for modeling skin conditions. The core elements of our method are the sequential transplantation of fibroblasts followed by keratinocytes seeded into a fibrin-based hydrogel in a silicone chamber. We optimized the fibrin gel formulation, timing for gel polymerization in vivo, cell culture conditions, and seeding density to make a robust and efficient grafting protocol. Using this approach, we can successfully engraft as few as 1.0 × 106 fresh and 2.0 × 106 frozen-then-thawed keratinocytes per 1.4 cm2 of the wound area. Additionally, it was concluded that a successful layer-by-layer engraftment of skin cells in vivo could be obtained without labor-intensive and costly methodologies such as bioprinting or engineering complex skin equivalents. Key features • Expands upon the conventional skin chamber assay method (Wang et al., 2000) to generate high-quality skin grafts using a minimal number of cultured skin cells. • The proposed approach allows the use of frozen-then-thawed keratinocytes and fibroblasts in surgical procedures. • This system holds promise for evaluating the functionality of skin cells derived from induced pluripotent stem cells and replicating various skin phenotypes. • The entire process, from thawing skin cells to establishing the graft, requires 54 days. Graphical overview.
Keywords: Fibrin-based hydrogel; Human skin equivalent; In vivo skin model; Multilayered skin graft; Regenerative medicine.
©Copyright : © 2024 The Authors; This is an open access article under the CC BY-NC license.
Conflict of interest statement
Competing interestsDRR has an equity interest in AVITA Medical. KB is an employee of AVITA Medical, and AH was an employee at the time the research was conducted. AVITA Medical may potentially benefit from the research findings presented here. Other authors do not have any potential conflicts of interest to declare.
Figures


Similar articles
-
Generation of a Full-Thickness Human Skin Equivalent on an Immunodeficient Mouse.Methods Mol Biol. 2020;2109:169-183. doi: 10.1007/7651_2019_236. Methods Mol Biol. 2020. PMID: 31119714 Free PMC article.
-
Bioprinting for Skin.Methods Mol Biol. 2020;2140:217-228. doi: 10.1007/978-1-0716-0520-2_14. Methods Mol Biol. 2020. PMID: 32207115
-
Three Dimensional Bioprinting of a Vascularized and Perfusable Skin Graft Using Human Keratinocytes, Fibroblasts, Pericytes, and Endothelial Cells.Tissue Eng Part A. 2020 Mar;26(5-6):227-238. doi: 10.1089/ten.TEA.2019.0201. Epub 2019 Dec 3. Tissue Eng Part A. 2020. PMID: 31672103 Free PMC article.
-
Translational stem cell therapy: vascularized skin grafts in skin repair and regeneration.J Transl Med. 2021 Feb 18;19(1):83. doi: 10.1186/s12967-021-02752-2. J Transl Med. 2021. PMID: 33602284 Free PMC article. Review.
-
Cellular Interaction of Human Skin Cells towards Natural Bioink via 3D-Bioprinting Technologies for Chronic Wound: A Comprehensive Review.Int J Mol Sci. 2022 Jan 1;23(1):476. doi: 10.3390/ijms23010476. Int J Mol Sci. 2022. PMID: 35008902 Free PMC article. Review.
References
-
- Cooper M. L., Andree C., Hansbrough J. F., Zapata-Sirvent R. L. and Spielvogel R. L.(1993). Direct comparison of a cultured composite skin substitute containing human keratinocytes and fibroblasts to an epidermal sheet graft containing human keratinocytes on athymic mice. J Invest Dermatol 101(6): 811-819. - PubMed
-
- Cristobal L., Asunsolo A., Sanchez J., Ortega M. A., Alvarez-Mon M., Garcia-Honduvilla N., Bujan J. and Maldonado A. A.(2021). Mouse Models for Human Skin Transplantation: A Systematic Review. Cells Tissues Organs 210(4): 250-259. - PubMed
-
- Del Rio M., Larcher F., Serrano F., Meana A., Munoz M., Garcia M., Munoz E., Martin C., Bernad A. and Jorcano J. L.(2002). A preclinical model for the analysis of genetically modified human skin in vivo. Hum Gene Ther 13(8): 959-968. - PubMed
-
- Escamez M. J., Garcia M., Larcher F., Meana A., Munoz E., Jorcano J. L. and Del Rio M.(2004). An in vivo model of wound healing in genetically modified skin-humanized mice. J Invest Dermatol 123(6): 1182-1191. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

