Alleviating effect of lavender (Lavandula angustifolia) and its major components on postherpetic pain: a randomized blinded controlled trial
- PMID: 38267936
- PMCID: PMC10807075
- DOI: 10.1186/s12906-024-04362-z
Alleviating effect of lavender (Lavandula angustifolia) and its major components on postherpetic pain: a randomized blinded controlled trial
Abstract
Background: Postherpetic neuralgia (PHN) causes severe pain which can lead to decreased quality-of-life. This study aimed to evaluate the effects of inhalation of lavender (Lavandula angustifolia) oil and its major components (linalool and linalyl acetate) on the pain in patients with PHN.
Methods: This study was performed at an outpatient clinic. Sixty-four patients with postherpetic neuralgia were randomly allocated to a control group (almond oil) or one of three experimental groups (lavender oil, linalool, or linalyl acetate diluted in almond oil at concentration of 1% v/v), and the participants inhaled the aroma by natural breathing. Quality, severity, and intensity of pain were measured before and after the intervention.
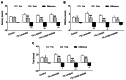
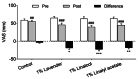
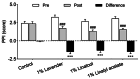
Results: Six patients discontinued the intervention for personal reasons; hence, data from 58 patients were analyzed (control group, n = 14; 1% lavender oil group, n = 15; 1% linalool, n = 15; 1% linalyl acetate, n = 14). Reduction in sensory pain was greater in the 1% lavender oil group, 1% linalool group, and 1% linalyl acetate group than in the control group (all P < 0.001). Reduction in affective pain was greater in the 1% lavender group (P < 0.001) and the 1% linalool group (P = 0.007) than in the control group. Decreases in pain severity and intensity were significantly greater in all three intervention groups than in the control group.
Conclusions: Inhalation of lavender oil and its major volatile components effectively reduced the quality, severity, and intensity of postherpetic pain, suggesting that lavender oil, linalool, and linalyl acetate may each be an effective intervention for reducing pain in patients with postherpetic neuralgia.
Trial registration: This study was retrospectively registered on the Clinical Research Information Service.
Registration number: KCT0007772, first registration 06/10/2022.
Keywords: Complementary and alternative medicine; Lavender; Linalool; Linalyl acetate; Postherpetic neuralgia.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Anti-psoriatic effect of Lavandula angustifolia essential oil and its major components linalool and linalyl acetate.J Ethnopharmacol. 2020 Oct 28;261:113127. doi: 10.1016/j.jep.2020.113127. Epub 2020 Jul 3. J Ethnopharmacol. 2020. PMID: 32623016
-
Essential Oil Content and Compositional Variability of Lavandula Species Cultivated in the Mid Hill Conditions of the Western Himalaya.Molecules. 2022 May 25;27(11):3391. doi: 10.3390/molecules27113391. Molecules. 2022. PMID: 35684332 Free PMC article.
-
Cytotoxicity of lavender oil and its major components to human skin cells.Cell Prolif. 2004 Jun;37(3):221-9. doi: 10.1111/j.1365-2184.2004.00307.x. Cell Prolif. 2004. PMID: 15144499 Free PMC article.
-
Intraplantar injection of bergamot essential oil into the mouse hindpaw: effects on capsaicin-induced nociceptive behaviors.Int Rev Neurobiol. 2009;85:237-48. doi: 10.1016/S0074-7742(09)85018-6. Int Rev Neurobiol. 2009. PMID: 19607974 Review.
-
Lavender Plant: Farming and Health Benefits.Curr Mol Med. 2024;24(6):702-711. doi: 10.2174/1566524023666230518114027. Curr Mol Med. 2024. PMID: 37202896 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

