Tumor-Associated Macrophage Targeting of Nanomedicines in Cancer Therapy
- PMID: 38258072
- PMCID: PMC10819517
- DOI: 10.3390/pharmaceutics16010061
Tumor-Associated Macrophage Targeting of Nanomedicines in Cancer Therapy
Abstract
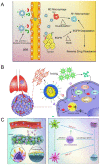
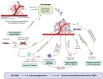
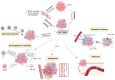
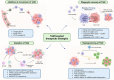
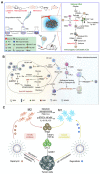
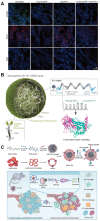
The tumor microenvironment (TME) is pivotal in tumor growth and metastasis, aligning with the "Seed and Soil" theory. Within the TME, tumor-associated macrophages (TAMs) play a central role, profoundly influencing tumor progression. Strategies targeting TAMs have surfaced as potential therapeutic avenues, encompassing interventions to block TAM recruitment, eliminate TAMs, reprogram M2 TAMs, or bolster their phagocytic capabilities via specific pathways. Nanomaterials including inorganic materials, organic materials for small molecules and large molecules stand at the forefront, presenting significant opportunities for precise targeting and modulation of TAMs to enhance therapeutic efficacy in cancer treatment. This review provides an overview of the progress in designing nanoparticles for interacting with and influencing the TAMs as a significant strategy in cancer therapy. This comprehensive review presents the role of TAMs in the TME and various targeting strategies as a promising frontier in the ever-evolving field of cancer therapy. The current trends and challenges associated with TAM-based therapy in cancer are presented.
Keywords: nanomedicine; suppressive immune environment; targeted delivery systems; tumor proliferation and metastasis; tumor-associated macrophage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Functionalized Nanoparticles Targeting Tumor-Associated Macrophages as Cancer Therapy.Pharmaceutics. 2021 Oct 13;13(10):1670. doi: 10.3390/pharmaceutics13101670. Pharmaceutics. 2021. PMID: 34683963 Free PMC article. Review.
-
Targeting tumor-associated macrophages in the tumor microenvironment.Oncol Lett. 2020 Nov;20(5):234. doi: 10.3892/ol.2020.12097. Epub 2020 Sep 14. Oncol Lett. 2020. PMID: 32968456 Free PMC article. Review.
-
Nanomaterials in modulating tumor-associated macrophages and enhancing immunotherapy.J Mater Chem B. 2024 May 22;12(20):4809-4823. doi: 10.1039/d4tb00230j. J Mater Chem B. 2024. PMID: 38695349 Review.
-
Nanomedicine-based cancer immunotherapies developed by reprogramming tumor-associated macrophages.Nanoscale. 2021 Mar 12;13(9):4705-4727. doi: 10.1039/d0nr08050k. Nanoscale. 2021. PMID: 33625411 Review.
-
Macrophage targeting in cancer.Ann N Y Acad Sci. 2021 Sep;1499(1):18-41. doi: 10.1111/nyas.14377. Epub 2020 May 22. Ann N Y Acad Sci. 2021. PMID: 32445205 Review.
Cited by
-
Tumor-associated macrophages within the immunological milieu: An emerging focal point for therapeutic intervention.Heliyon. 2024 Aug 23;10(17):e36839. doi: 10.1016/j.heliyon.2024.e36839. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39281573 Free PMC article. Review.
-
Targeting tumor-associated macrophages with nanocarrier-based treatment for breast cancer: A step toward developing innovative anti-cancer therapeutics.Heliyon. 2024 Aug 30;10(18):e37217. doi: 10.1016/j.heliyon.2024.e37217. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39309874 Free PMC article. Review.
-
Physiologically-based pharmacokinetic model for evaluating gender-specific exposures of N-nitrosodimethylamine (NDMA).Arch Toxicol. 2024 Mar;98(3):821-835. doi: 10.1007/s00204-023-03652-8. Epub 2023 Dec 21. Arch Toxicol. 2024. PMID: 38127128
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

