1,2,4-Triazole-Tethered Indolinones as New Cancer-Fighting Small Molecules Targeting VEGFR-2: Synthesis, Biological Evaluations and Molecular Docking
- PMID: 38256914
- PMCID: PMC10820444
- DOI: 10.3390/ph17010081
1,2,4-Triazole-Tethered Indolinones as New Cancer-Fighting Small Molecules Targeting VEGFR-2: Synthesis, Biological Evaluations and Molecular Docking
Abstract
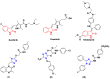
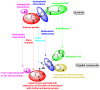
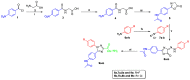
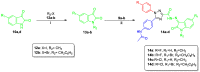
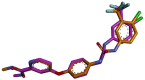
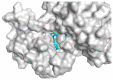
Targeting the VEGFR-2 signaling pathway is an inveterate approach toward combating pancreatic and hepatocellular cancers. Based on Sunitinib, the FDA-approved VEGFR-2 inhibitor, novel indolin-2-one-triazole hybrids were designed and synthesized as anti-hepatocellular and anti-pancreatic cancer agents with VEGFR-2 inhibitory activity. All the targeted compounds were assessed for their anti-cancer activity, revealing IC50 values extending from 0.17 to 4.29 µM for PANC1 and 0.58 to 4.49 µM for HepG2 cell lines. An extensive SAR study was conducted to explore the effect of different substituents along with N-alkylation. The potent anti-cancer analogs 11d, 11e, 11g, 11k and 14c were evaluated for their VEGFR-2 inhibitory actions, where their IC50 values ranged from 16.3 to 119.6 nM compared to Sorafenib, which revealed an IC50 of 29.7 nM, having compound 11d as the most active analog. An in silico ADME study was performed to confirm the drug-likeness of the synthesized compounds. Finally, molecular docking simulation was conducted for the most potent VEGFR-2 inhibitor (11d), demonstrating the strong binding with the vital amino acid residues of the VEGFR-2 ATP binding site.
Keywords: angiogenesis; anti-cancer agents; molecular modeling; synthesis; tail approach.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
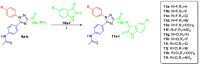


Similar articles
-
Discovery of novel indolyl-1,2,4-triazole hybrids as potent vascular endothelial growth factor receptor-2 (VEGFR-2) inhibitors with potential anti-renal cancer activity.Bioorg Chem. 2020 Dec;105:104330. doi: 10.1016/j.bioorg.2020.104330. Epub 2020 Oct 1. Bioorg Chem. 2020. PMID: 33038552
-
Design, Synthesis, In Vitro Anti-cancer Activity, ADMET Profile and Molecular Docking of Novel Triazolo[3,4-a]phthalazine Derivatives Targeting VEGFR-2 Enzyme.Anticancer Agents Med Chem. 2018;18(8):1184-1196. doi: 10.2174/1871520618666180412123833. Anticancer Agents Med Chem. 2018. PMID: 29651967
-
Discovery of new 3-methylquinoxalines as potential anti-cancer agents and apoptosis inducers targeting VEGFR-2: design, synthesis, and in silico studies.J Enzyme Inhib Med Chem. 2021 Dec;36(1):1732-1750. doi: 10.1080/14756366.2021.1945591. J Enzyme Inhib Med Chem. 2021. PMID: 34325596 Free PMC article.
-
Targeting the interplay between MMP-2, CA II and VEGFR-2 via new sulfonamide-tethered isomeric triazole hybrids; Microwave-assisted synthesis, computational studies and evaluation.Bioorg Chem. 2022 Jul;124:105816. doi: 10.1016/j.bioorg.2022.105816. Epub 2022 Apr 16. Bioorg Chem. 2022. PMID: 35489270 Review.
-
Novel benzothiazole-based dual VEGFR-2/EGFR inhibitors targeting breast and liver cancers: Synthesis, cytotoxic activity, QSAR and molecular docking studies.Bioorg Med Chem Lett. 2022 Feb 15;58:128529. doi: 10.1016/j.bmcl.2022.128529. Epub 2022 Jan 7. Bioorg Med Chem Lett. 2022. PMID: 35007724 Review.
Cited by
-
Identification of 2-(N-aryl-1,2,3-triazol-4-yl) quinoline derivatives as antitubercular agents endowed with InhA inhibitory activity.Front Chem. 2024 Aug 7;12:1424017. doi: 10.3389/fchem.2024.1424017. eCollection 2024. Front Chem. 2024. PMID: 39170867 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

