Molecular and Cellular Mechanisms Underlying the Cardiac Hypertrophic and Pro-Remodelling Effects of Leptin
- PMID: 38256208
- PMCID: PMC10816997
- DOI: 10.3390/ijms25021137
Molecular and Cellular Mechanisms Underlying the Cardiac Hypertrophic and Pro-Remodelling Effects of Leptin
Abstract
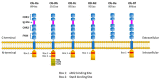
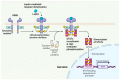
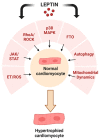
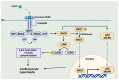
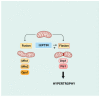
Since its initial discovery in 1994, the adipokine leptin has received extensive interest as an important satiety factor and regulator of energy expenditure. Although produced primarily by white adipocytes, leptin can be synthesized by numerous tissues including those comprising the cardiovascular system. Cardiovascular function can thus be affected by locally produced leptin via an autocrine or paracrine manner but also by circulating leptin. Leptin exerts its effects by binding to and activating specific receptors, termed ObRs or LepRs, belonging to the Class I cytokine family of receptors of which six isoforms have been identified. Although all ObRs have identical intracellular domains, they differ substantially in length in terms of their extracellular domains, which determine their ability to activate cell signalling pathways. The most important of these receptors in terms of biological effects of leptin is the so-called long form (ObRb), which possesses the complete intracellular domain linked to full cell signalling processes. The heart has been shown to express ObRb as well as to produce leptin. Leptin exerts numerous cardiac effects including the development of hypertrophy likely through a number of cell signaling processes as well as mitochondrial dynamics, thus demonstrating substantial complex underlying mechanisms. Here, we discuss mechanisms that potentially mediate leptin-induced cardiac pathological hypertrophy, which may contribute to the development of heart failure.
Keywords: autophagy; cardiac hypertrophy and remodelling; heart failure; intracellular signalling; leptin; mitochondrial dynamics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
The potential contribution of circulating and locally produced leptin to cardiac hypertrophy and failure.Can J Physiol Pharmacol. 2013 Nov;91(11):883-8. doi: 10.1139/cjpp-2013-0057. Epub 2013 May 8. Can J Physiol Pharmacol. 2013. PMID: 24117255 Review.
-
Leptin as a cardiac hypertrophic factor: a potential target for therapeutics.Trends Cardiovasc Med. 2007 Aug;17(6):206-11. doi: 10.1016/j.tcm.2007.06.001. Trends Cardiovasc Med. 2007. PMID: 17662916 Review.
-
Leptin as a cardiac pro-hypertrophic factor and its potential role in the development of heart failure.Curr Pharm Des. 2014;20(4):646-51. doi: 10.2174/13816128113199990023. Curr Pharm Des. 2014. PMID: 23688017 Review.
-
Identification of functional leptin receptors expressed in ventricular mitochondria.Mol Cell Biochem. 2015 Oct;408(1-2):155-62. doi: 10.1007/s11010-015-2491-2. Epub 2015 Jun 30. Mol Cell Biochem. 2015. PMID: 26122392
-
Leptin signalling reduces the severity of cardiac dysfunction and remodelling after chronic ischaemic injury.Cardiovasc Res. 2008 Jan;77(1):54-63. doi: 10.1093/cvr/cvm023. Epub 2007 Sep 20. Cardiovasc Res. 2008. PMID: 18006469
Cited by
-
Adipokines and their potential impacts on susceptibility to myocardial ischemia/reperfusion injury in diabetes.Lipids Health Dis. 2024 Nov 13;23(1):372. doi: 10.1186/s12944-024-02357-w. Lipids Health Dis. 2024. PMID: 39538244 Free PMC article. Review.
References
-
- Maffei M., Fei H., Lee G.H., Dani C., Leroy P., Zhang Y., Proenca R., Negrel R., Ailhaud G., Friedman J.M. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc. Natl. Acad. Sci. USA. 1995;92:6957–6960. doi: 10.1073/pnas.92.15.6957. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

