The Role of MicroRNA, Long Non-Coding RNA and Circular RNA in the Pathogenesis of Polycystic Ovary Syndrome: A Literature Review
- PMID: 38255975
- PMCID: PMC10815174
- DOI: 10.3390/ijms25020903
The Role of MicroRNA, Long Non-Coding RNA and Circular RNA in the Pathogenesis of Polycystic Ovary Syndrome: A Literature Review
Abstract
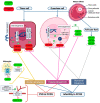
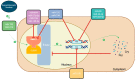
Polycystic ovary syndrome (PCOS) is the most common endocrine-metabolic disease in females of reproductive age, affecting 4-20% of pre-menopausal women worldwide. MicroRNAs (miRNAs) are endogenous, single-stranded, non-coding, regulatory ribonucleic acid molecules found in eukaryotic cells. Abnormal miRNA expression has been associated with several diseases and could possibly explain their underlying pathophysiology. MiRNAs have been extensively studied for their potential diagnostic, prognostic, and therapeutic uses in many diseases, such as type 2 diabetes, obesity, cardiovascular disease, PCOS, and endometriosis. In women with PCOS, miRNAs were found to be abnormally expressed in theca cells, follicular fluid, granulosa cells, peripheral blood leukocytes, serum, and adipose tissue when compared to those without PCOS, making miRNAs a useful potential biomarker for the disease. Key pathways involved in PCOS, such as folliculogenesis, steroidogenesis, and cellular adhesion, are regulated by miRNA. This also highlights their importance as potential prognostic markers. In addition, recent evidence suggests a role for miRNAs in regulating the circadian rhythm (CR). CR is crucial for regulating reproduction through the various functions of the hypothalamic-pituitary-gonadal (HPG) axis and the ovaries. A disordered CR affects reproductive outcomes by inducing insulin resistance, oxidative stress, and systemic inflammation. Moreover, miRNAs were demonstrated to interact with lncRNA and circRNAs, which are thought to play a role in the pathogenesis of PCOS. This review discusses what is currently understood about miRNAs in PCOS, the cellular pathways involved, and their potential role as biomarkers and therapeutic targets.
Keywords: biomarkers; cardiovascular disease; circadian rhythm; circular RNA (circRNA); infertility; insulin resistance; long coding RNA (lncRNA); microRNA (miRNAs); polycystic ovary syndrome (PCOS); therapeutic targets.
Conflict of interest statement
None of the authors have any conflict of interest to declare.
Figures
Similar articles
-
Non-coding RNAs in polycystic ovary syndrome: a systematic review and meta-analysis.Reprod Biol Endocrinol. 2021 Jan 14;19(1):10. doi: 10.1186/s12958-020-00687-9. Reprod Biol Endocrinol. 2021. PMID: 33446212 Free PMC article.
-
The role of MiRNA in polycystic ovary syndrome (PCOS).Gene. 2019 Jul 20;706:91-96. doi: 10.1016/j.gene.2019.04.082. Epub 2019 May 1. Gene. 2019. PMID: 31054362 Review.
-
Circular RNAs: Emerging Modulators in the Pathophysiology of Polycystic Ovary Syndrome and their Clinical Implications.Curr Mol Med. 2024;24(2):153-166. doi: 10.2174/1566524023666230110151155. Curr Mol Med. 2024. PMID: 36627779 Review.
-
miRNAs as a novel clinical biomarker and therapeutic targets in polycystic ovary syndrome (PCOS): A review.Life Sci. 2020 Oct 15;259:118174. doi: 10.1016/j.lfs.2020.118174. Epub 2020 Aug 1. Life Sci. 2020. PMID: 32745529 Review.
-
Characterization of microRNA profile in human cumulus granulosa cells: Identification of microRNAs that regulate Notch signaling and are associated with PCOS.Mol Cell Endocrinol. 2015 Mar 15;404:26-36. doi: 10.1016/j.mce.2015.01.030. Epub 2015 Jan 23. Mol Cell Endocrinol. 2015. PMID: 25622783
Cited by
-
LncRNA PKD1P6 modulates ovarian granulosa cell survival of hyperandrogenic polycystic ovary syndrome by targeting miR-135b-5p and inhibiting ERK1/2 signaling.Heliyon. 2024 Aug 14;10(16):e36321. doi: 10.1016/j.heliyon.2024.e36321. eCollection 2024 Aug 30. Heliyon. 2024. PMID: 39253226 Free PMC article.
-
Non-coding RNA: A key regulator in the Glutathione-GPX4 pathway of ferroptosis.Noncoding RNA Res. 2024 May 20;9(4):1222-1234. doi: 10.1016/j.ncrna.2024.05.007. eCollection 2024 Dec. Noncoding RNA Res. 2024. PMID: 39036600 Free PMC article. Review.
-
circ_SMA4 promotes gastrointestinal stromal tumors malignant progression by sponging miR-494-3p/KIT axis and activating JAK/STAT pathway.Sci Rep. 2024 Sep 24;14(1):22004. doi: 10.1038/s41598-024-73393-w. Sci Rep. 2024. PMID: 39317735 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

