Modulation of Tau Pathology in Alzheimer's Disease by Dietary Bioactive Compounds
- PMID: 38255905
- PMCID: PMC10815728
- DOI: 10.3390/ijms25020831
Modulation of Tau Pathology in Alzheimer's Disease by Dietary Bioactive Compounds
Abstract
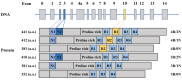
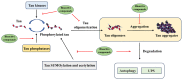
Tau is a microtubule-associated protein essential for microtubule assembly and stability in neurons. The abnormal intracellular accumulation of tau aggregates is a major characteristic of brains from patients with Alzheimer's disease (AD) and other tauopathies. In AD, the presence of neurofibrillary tangles (NFTs), which is composed of hyperphosphorylated tau protein, is positively correlated with the severity of the cognitive decline. Evidence suggests that the accumulation and aggregation of tau cause synaptic dysfunction and neuronal degeneration. Thus, the prevention of abnormal tau phosphorylation and elimination of tau aggregates have been proposed as therapeutic strategies for AD. However, currently tau-targeting therapies for AD and other tauopathies are limited. A number of dietary bioactive compounds have been found to modulate the posttranslational modifications of tau, including phosphorylation, small ubiquitin-like modifier (SUMO) mediated modification (SUMOylation) and acetylation, as well as inhibit tau aggregation and/or promote tau degradation. The advantages of using these dietary components over synthetic substances in AD prevention and intervention are their safety and accessibility. This review summarizes the mechanisms leading to tau pathology in AD and highlights the effects of bioactive compounds on the hyperphosphorylation, aggregation and clearance of tau protein. The potential of using these bioactive compounds for AD prevention and intervention is also discussed.
Keywords: Alzheimer’s disease; dietary bioactive compounds; tau pathology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Hyperphosphorylated tau (p-tau) and drug discovery in the context of Alzheimer's disease and related tauopathies.Drug Discov Today. 2023 Mar;28(3):103487. doi: 10.1016/j.drudis.2023.103487. Epub 2023 Jan 9. Drug Discov Today. 2023. PMID: 36634842 Free PMC article. Review.
-
Novel drugs affecting tau behavior in the treatment of Alzheimer's disease and tauopathies.Curr Alzheimer Res. 2011 Sep;8(6):678-85. doi: 10.2174/156720511796717122. Curr Alzheimer Res. 2011. PMID: 21605038 Review.
-
Tau Protein Hyperphosphorylation and Aggregation in Alzheimer's Disease and Other Tauopathies, and Possible Neuroprotective Strategies.Biomolecules. 2016 Jan 6;6(1):6. doi: 10.3390/biom6010006. Biomolecules. 2016. PMID: 26751493 Free PMC article. Review.
-
Tau-directed drug discovery for Alzheimer's disease and related tauopathies: a focus on tau assembly inhibitors.Exp Neurol. 2010 Jun;223(2):304-10. doi: 10.1016/j.expneurol.2009.08.031. Epub 2009 Sep 8. Exp Neurol. 2010. PMID: 19744482 Free PMC article. Review.
-
Acetylated tau, a novel pathological signature in Alzheimer's disease and other tauopathies.Brain. 2012 Mar;135(Pt 3):807-18. doi: 10.1093/brain/aws013. Brain. 2012. PMID: 22366796 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

