Proline Dehydrogenase (PRODH) Is Expressed in Lung Adenocarcinoma and Modulates Cell Survival and 3D Growth by Inducing Cellular Senescence
- PMID: 38255788
- PMCID: PMC10815008
- DOI: 10.3390/ijms25020714
Proline Dehydrogenase (PRODH) Is Expressed in Lung Adenocarcinoma and Modulates Cell Survival and 3D Growth by Inducing Cellular Senescence
Abstract
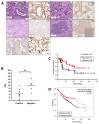
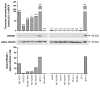
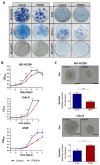
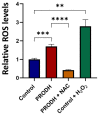
The identification of markers for early diagnosis, prognosis, and improvement of therapeutic options represents an unmet clinical need to increase survival in Non-Small Cell Lung Cancer (NSCLC), a neoplasm still characterized by very high incidence and mortality. Here, we investigated whether proline dehydrogenase (PRODH), a mitochondrial flavoenzyme catalyzing the key step in proline degradation, played a role in NSCLC tumorigenesis. PRODH expression was investigated by immunohistochemistry; digital PCR, quantitative PCR, immunoblotting, measurement of reactive oxygen species (ROS), and functional cellular assays were carried out. PRODH expression was found in the majority of lung adenocarcinomas (ADCs). Patients with PRODH-positive tumors had better cancer-free specific and overall survival compared to those with negative tumors. Ectopic modulation of PRODH expression in NCI-H1299 and the other tested lung ADC cell lines decreased cell survival. Moreover, cell proliferation curves showed delayed growth in NCI-H1299, Calu-6 and A549 cell lines when PRODH-expressing clones were compared to control clones. The 3D growth in soft agar was also impaired in the presence of PRODH. PRODH increased reactive oxygen species production and induced cellular senescence in the NCI-H1299 cell line. This study supports a role of PRODH in decreasing survival and growth of lung ADC cells by inducing cellular senescence.
Keywords: adenocarcinoma cell lines; cell-based assays; cellular senescence; immunohistochemical analysis; lung cancer; proline dehydrogenase.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Proline dehydrogenase promotes senescence through the generation of reactive oxygen species.J Cell Sci. 2017 Apr 15;130(8):1413-1420. doi: 10.1242/jcs.196469. Epub 2017 Mar 6. J Cell Sci. 2017. PMID: 28264926
-
Proline dehydrogenase in cancer: apoptosis, autophagy, nutrient dependency and cancer therapy.Amino Acids. 2021 Dec;53(12):1891-1902. doi: 10.1007/s00726-021-03032-5. Epub 2021 Jul 20. Amino Acids. 2021. PMID: 34283310 Review.
-
Proline dehydrogenase (oxidase) in cancer.Biofactors. 2012 Nov-Dec;38(6):398-406. doi: 10.1002/biof.1036. Epub 2012 Aug 8. Biofactors. 2012. PMID: 22886911 Free PMC article. Review.
-
Nonsteroidal Anti-Inflammatory Drugs as PPARγ Agonists Can Induce PRODH/POX-Dependent Apoptosis in Breast Cancer Cells: New Alternative Pathway in NSAID-Induced Apoptosis.Int J Mol Sci. 2022 Jan 28;23(3):1510. doi: 10.3390/ijms23031510. Int J Mol Sci. 2022. PMID: 35163433 Free PMC article.
-
Cancer progression is mediated by proline catabolism in non-small cell lung cancer.Oncogene. 2020 Mar;39(11):2358-2376. doi: 10.1038/s41388-019-1151-5. Epub 2020 Jan 7. Oncogene. 2020. PMID: 31911619
Cited by
-
Exploring the dynamic three-dimensional chromatin architecture and transcriptional landscape in goose liver tissues underlying metabolic adaptations induced by a high-fat diet.J Anim Sci Biotechnol. 2024 May 2;15(1):60. doi: 10.1186/s40104-024-01016-5. J Anim Sci Biotechnol. 2024. PMID: 38693536 Free PMC article.
References
-
- Wilde L., Roche M., Domingo-Vidal M., Tanson K., Philp N., Curry J., Martinez-Outschoorn U. Metabolic coupling and the Reverse Warburg Effect in cancer: Implications for novel biomarker and anticancer agent development. Semin. Oncol. 2017;44:198–203. doi: 10.1053/j.seminoncol.2017.10.004. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

