Alveolar Organoids in Lung Disease Modeling
- PMID: 38254715
- PMCID: PMC10813493
- DOI: 10.3390/biom14010115
Alveolar Organoids in Lung Disease Modeling
Abstract
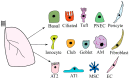
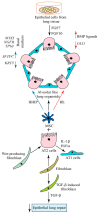
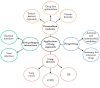
Lung organoids display a tissue-specific functional phenomenon and mimic the features of the original organ. They can reflect the properties of the cells, such as morphology, polarity, proliferation rate, gene expression, and genomic profile. Alveolar type 2 (AT2) cells have a stem cell potential in the adult lung. They produce and secrete pulmonary surfactant and proliferate to restore the epithelium after damage. Therefore, AT2 cells are used to generate alveolar organoids and can recapitulate distal lung structures. Also, AT2 cells in human-induced pluripotent stem cell (iPSC)-derived alveolospheres express surfactant proteins and other factors, indicating their application as suitable models for studying cell-cell interactions. Recently, they have been utilized to define mechanisms of disease development, such as COVID-19, lung cancer, idiopathic pulmonary fibrosis, and chronic obstructive pulmonary disease. In this review, we show lung organoid applications in various pulmonary diseases, drug screening, and personalized medicine. In addition, stem cell-based therapeutics and approaches relevant to lung repair were highlighted. We also described the signaling pathways and epigenetic regulation of lung regeneration. It is critical to identify novel regulators of alveolar organoid generations to promote lung repair in pulmonary diseases.
Keywords: AT2 cells; alveolar organoids; diseases; lung; regeneration.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
In Vitro Disease Modeling of Hermansky-Pudlak Syndrome Type 2 Using Human Induced Pluripotent Stem Cell-Derived Alveolar Organoids.Stem Cell Reports. 2019 Mar 5;12(3):431-440. doi: 10.1016/j.stemcr.2019.01.014. Epub 2019 Feb 14. Stem Cell Reports. 2019. PMID: 30773483 Free PMC article.
-
iPSC-derived mesenchymal cells that support alveolar organoid development.Cell Rep Methods. 2022 Sep 19;2(10):100314. doi: 10.1016/j.crmeth.2022.100314. eCollection 2022 Oct 24. Cell Rep Methods. 2022. PMID: 36313800 Free PMC article.
-
Paracrine Regulation of Alveolar Epithelial Damage and Repair Responses by Human Lung-Resident Mesenchymal Stromal Cells.Cells. 2021 Oct 23;10(11):2860. doi: 10.3390/cells10112860. Cells. 2021. PMID: 34831082 Free PMC article.
-
Modeling of Respiratory Diseases Evolving with Fibrosis from Organoids Derived from Human Pluripotent Stem Cells.Int J Mol Sci. 2023 Feb 23;24(5):4413. doi: 10.3390/ijms24054413. Int J Mol Sci. 2023. PMID: 36901843 Free PMC article. Review.
-
Human pluripotent stem cell-derived lung organoids: Potential applications in development and disease modeling.Wiley Interdiscip Rev Dev Biol. 2021 Nov;10(6):e399. doi: 10.1002/wdev.399. Epub 2020 Nov 3. Wiley Interdiscip Rev Dev Biol. 2021. PMID: 33145915 Review.
Cited by
-
Causal Relationship Between Gut Microbiota and Chronic Obstructive Pulmonary Disease: A Bidirectional Two-Sample Mendelian Randomization Study.Int J Chron Obstruct Pulmon Dis. 2024 Sep 2;19:1957-1969. doi: 10.2147/COPD.S464917. eCollection 2024. Int J Chron Obstruct Pulmon Dis. 2024. PMID: 39247666 Free PMC article.
References
-
- Liu T., Zhou C., Shao Y., Xiong Z., Weng D., Pang Y., Sun W. Construction and Application of in vitro Alveolar Models Based on 3D Printing Technology. Chin. J. Mech. Eng. Addit. Manuf. Front. 2022;1:100025. doi: 10.1016/j.cjmeam.2022.100025. - DOI
-
- Kobayashi Y., Tata A., Konkimalla A., Katsura H., Lee R.F., Ou J., Banovich N.E., Kropski J.A., Tata P.R. Persistence of a regeneration-associated, transitional alveolar epithelial cell state in pulmonary fibrosis. Nat. Cell Biol. 2020;22:934–946. doi: 10.1038/s41556-020-0542-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

