Wheat Bran Polyphenols Ameliorate DSS-Induced Ulcerative Colitis in Mice by Suppressing MAPK/NF-κB Inflammasome Pathways and Regulating Intestinal Microbiota
- PMID: 38254526
- PMCID: PMC10814686
- DOI: 10.3390/foods13020225
Wheat Bran Polyphenols Ameliorate DSS-Induced Ulcerative Colitis in Mice by Suppressing MAPK/NF-κB Inflammasome Pathways and Regulating Intestinal Microbiota
Abstract
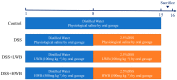
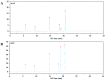
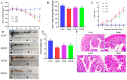
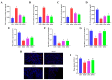
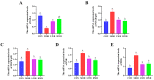
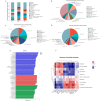
Wheat bran (WB) is the primary by-product of wheat processing and contains a high concentration of bioactive substances such as polyphenols. This study analyzed the qualitative and quantitative components of polyphenols in wheat bran and their effects on ulcerative colitis (UC) using the dextran sulfate sodium (DSS)-induced colitis model in mice. The potential mechanism of wheat bran polyphenols (WBP) was also examined. Our findings indicate that the main polyphenol constituents of WBP were phenolic acids, including vanillic acid, ferulic acid, caffeic acid, gallic acid, and protocatechuic acid. Furthermore, WBP exerted remarkable protective effects against experimental colitis. This was achieved by reducing the severity of colitis and improving colon morphology. Additionally, WBP suppressed colonic inflammation via upregulation of the anti-inflammatory cytokine IL-10 and downregulation of pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) in colon tissues. Mechanistically, WBP ameliorated DSS-induced colitis in mice by inhibiting activation of the MAPK/NF-κB pathway. In addition, microbiome analysis results suggested that WBP modulated the alteration of gut microbiota caused by DSS, with an enhancement in the ratio of Firmicutes/Bacteroidetes and adjustments in the number of Helicobacter, Escherichia-Shigella, Akkermansia, Lactobacillus, Lachnospiraceae_NK4A136_group at the genus level. To conclude, the findings showed that WBP has excellent prospects in reducing colonic inflammation in UC mice.
Keywords: gut microbiota; inflammation; intestinal barrier function; ulcerative colitis; wheat bran polyphenols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
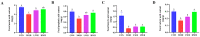

Similar articles
-
Thyme (Thymus vulgaris L.) polyphenols ameliorate DSS-induced ulcerative colitis of mice by mitigating intestinal barrier damage, regulating gut microbiota, and suppressing TLR4/NF-κB-NLRP3 inflammasome pathways.Food Funct. 2023 Jan 23;14(2):1113-1132. doi: 10.1039/d2fo02523j. Food Funct. 2023. PMID: 36594593
-
2,3,5,4'-Tetrahydroxystilbene-2-O-β-D-glucoside, a major bioactive component from Polygoni multiflori Radix (Heshouwu) suppresses DSS induced acute colitis in BALb/c mice by modulating gut microbiota.Biomed Pharmacother. 2021 May;137:111420. doi: 10.1016/j.biopha.2021.111420. Epub 2021 Feb 23. Biomed Pharmacother. 2021. PMID: 33761623
-
Protective effect of synbiotic combination of Lactobacillus plantarum SC-5 and olive oil extract tyrosol in a murine model of ulcerative colitis.J Transl Med. 2024 Mar 25;22(1):308. doi: 10.1186/s12967-024-05026-9. J Transl Med. 2024. PMID: 38528541 Free PMC article.
-
Pulsatilla decoction improves DSS-induced colitis via modulation of fecal-bacteria-related short-chain fatty acids and intestinal barrier integrity.J Ethnopharmacol. 2023 Jan 10;300:115741. doi: 10.1016/j.jep.2022.115741. Epub 2022 Sep 24. J Ethnopharmacol. 2023. PMID: 36162543
-
Painong-San extract alleviates dextran sulfate sodium-induced colitis in mice by modulating gut microbiota, restoring intestinal barrier function and attenuating TLR4/NF-κB signaling cascades.J Pharm Biomed Anal. 2022 Feb 5;209:114529. doi: 10.1016/j.jpba.2021.114529. Epub 2021 Dec 10. J Pharm Biomed Anal. 2022. PMID: 34915325
Cited by
-
Fabrication of the Rapid Self-Assembly Hydrogels Loaded with Luteolin: Their Structural Characteristics and Protection Effect on Ulcerative Colitis.Foods. 2024 Apr 4;13(7):1105. doi: 10.3390/foods13071105. Foods. 2024. PMID: 38611409 Free PMC article.
-
Polygonatum sibiricum Saponin Prevents Immune Dysfunction and Strengthens Intestinal Mucosal Barrier Function in Cyclophosphamide-Induced Immunosuppressed BALB/c Mice.Foods. 2024 Mar 19;13(6):934. doi: 10.3390/foods13060934. Foods. 2024. PMID: 38540924 Free PMC article.
References
-
- Zou M., Wang Y., Liu Y., Xiong S., Zhang L., Wang J. Huangshan Floral Mushroom Polysaccharide Ameliorates Dextran Sulfate Sodium-Induced Colitis in Mice by Modulating Th17/Treg Balance in a Gut Microbiota-Dependent Manner. Mol. Nutr. Food Res. 2023;67:2200408. doi: 10.1002/mnfr.202200408. - DOI - PubMed
-
- Zhou Z., He W., Tian H., Zhan P., Liu J. Thyme (Thymus vulgaris L.) polyphenols ameliorate DSS-induced ulcera-tive colitis of mice by mitigating intestinal barrier damage, regulating gut microbiota, and suppressing TLR4/NF-κB-NLRP3 inflammasome pathways. Food Funct. 2023;14:1113–1132. doi: 10.1039/D2FO02523J. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

