Crosstalk between colorectal CSCs and immune cells in tumorigenesis, and strategies for targeting colorectal CSCs
- PMID: 38254219
- PMCID: PMC10802076
- DOI: 10.1186/s40164-024-00474-x
Crosstalk between colorectal CSCs and immune cells in tumorigenesis, and strategies for targeting colorectal CSCs
Abstract
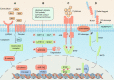
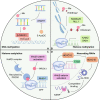
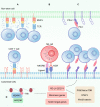
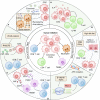
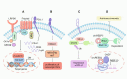
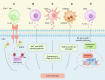
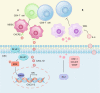
Cancer immunotherapy has emerged as a promising strategy in the treatment of colorectal cancer, and relapse after tumor immunotherapy has attracted increasing attention. Cancer stem cells (CSCs), a small subset of tumor cells with self-renewal and differentiation capacities, are resistant to traditional therapies such as radiotherapy and chemotherapy. Recently, CSCs have been proven to be the cells driving tumor relapse after immunotherapy. However, the mutual interactions between CSCs and cancer niche immune cells are largely uncharacterized. In this review, we focus on colorectal CSCs, CSC-immune cell interactions and CSC-based immunotherapy. Colorectal CSCs are characterized by robust expression of surface markers such as CD44, CD133 and Lgr5; hyperactivation of stemness-related signaling pathways, such as the Wnt/β-catenin, Hippo/Yap1, Jak/Stat and Notch pathways; and disordered epigenetic modifications, including DNA methylation, histone modification, chromatin remodeling, and noncoding RNA action. Moreover, colorectal CSCs express abnormal levels of immune-related genes such as MHC and immune checkpoint molecules and mutually interact with cancer niche cells in multiple tumorigenesis-related processes, including tumor initiation, maintenance, metastasis and drug resistance. To date, many therapies targeting CSCs have been evaluated, including monoclonal antibodies, antibody‒drug conjugates, bispecific antibodies, tumor vaccines adoptive cell therapy, and small molecule inhibitors. With the development of CSC-/niche-targeting technology, as well as the integration of multidisciplinary studies, novel therapies that eliminate CSCs and reverse their immunosuppressive microenvironment are expected to be developed for the treatment of solid tumors, including colorectal cancer.
Keywords: Colorectal cancer stem cells; Immune Cells; Immunotherapy; Targeting cancer stem cells; Tumor immune microenvironment.
© 2024. The Author(s).
Conflict of interest statement
All authors declare that they have no conflicts of interest affecting this work.
Figures
Similar articles
-
Update on immune-based therapy strategies targeting cancer stem cells.Cancer Med. 2023 Sep;12(18):18960-18980. doi: 10.1002/cam4.6520. Epub 2023 Sep 12. Cancer Med. 2023. PMID: 37698048 Free PMC article. Review.
-
Cancer stem cells and tumorigenesis.Biophys Rep. 2018;4(4):178-188. doi: 10.1007/s41048-018-0062-2. Epub 2018 Aug 29. Biophys Rep. 2018. PMID: 30310855 Free PMC article. Review.
-
Cancer stem cells (CSCs) in cancer progression and therapy.J Cell Physiol. 2019 Jun;234(6):8381-8395. doi: 10.1002/jcp.27740. Epub 2018 Nov 11. J Cell Physiol. 2019. PMID: 30417375 Review.
-
Concise Review: Targeting Cancer Stem Cells Using Immunologic Approaches.Stem Cells. 2015 Jul;33(7):2085-92. doi: 10.1002/stem.2039. Epub 2015 May 13. Stem Cells. 2015. PMID: 25873269 Free PMC article. Review.
-
Advances in Therapeutic Targeting of Cancer Stem Cells within the Tumor Microenvironment: An Updated Review.Cells. 2020 Aug 13;9(8):1896. doi: 10.3390/cells9081896. Cells. 2020. PMID: 32823711 Free PMC article. Review.
Cited by
-
Mechanism of action of miR-15a-5p and miR-152-3p in paraquat-induced pulmonary fibrosis through Wnt/β-catenin signaling mediation.PeerJ. 2024 Jul 8;12:e17662. doi: 10.7717/peerj.17662. eCollection 2024. PeerJ. 2024. PMID: 38993979 Free PMC article. Review.
-
Endoplasmic Reticulum Membrane Protein Complex Regulates Cancer Stem Cells and is Associated with Sorafenib Resistance in Hepatocellular Carcinoma.J Hepatocell Carcinoma. 2024 Aug 9;11:1519-1539. doi: 10.2147/JHC.S474343. eCollection 2024. J Hepatocell Carcinoma. 2024. PMID: 39139735 Free PMC article.
-
From mechanism to therapy: the journey of CD24 in cancer.Front Immunol. 2024 May 31;15:1401528. doi: 10.3389/fimmu.2024.1401528. eCollection 2024. Front Immunol. 2024. PMID: 38881902 Free PMC article. Review.
-
Unveiling the Dynamic Interplay between Cancer Stem Cells and the Tumor Microenvironment in Melanoma: Implications for Novel Therapeutic Strategies.Cancers (Basel). 2024 Aug 16;16(16):2861. doi: 10.3390/cancers16162861. Cancers (Basel). 2024. PMID: 39199632 Free PMC article. Review.
-
MicroRNA in prostate cancer: from biogenesis to applicative potential.BMC Urol. 2024 Nov 6;24(1):244. doi: 10.1186/s12894-024-01634-1. BMC Urol. 2024. PMID: 39506720 Free PMC article. Review.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

