Unexpected inhibition of the lipid kinase PIKfyve reveals an epistatic role for p38 MAPKs in endolysosomal fission and volume control
- PMID: 38253602
- PMCID: PMC10803372
- DOI: 10.1038/s41419-024-06423-0
Unexpected inhibition of the lipid kinase PIKfyve reveals an epistatic role for p38 MAPKs in endolysosomal fission and volume control
Abstract
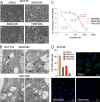
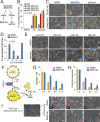
p38 mitogen-activated protein kinases (MAPKs) participate in autophagic signaling; and previous reports suggest that pyridinyl imidazole p38 MAPK inhibitors, including SB203580 and SB202190, induce cell death in some cancer cell-types through unrestrained autophagy. Subsequent studies, however, have suggested that the associated cytoplasmic vacuolation resulted from off-target inhibition of an unidentified enzyme. Herein, we report that SB203580-induced vacuolation is rapid, reversible, and relies on the class III phosphatidylinositol 3-kinase (PIK3C3) complex and the production of phosphatidylinositol 3-phosphate [PI(3)P] but not on autophagy per se. Rather, vacuolation resulted from the accumulation of Rab7 on late endosome and lysosome (LEL) membranes, combined with an osmotic imbalance that triggered severe swelling in these organelles. Inhibition of PIKfyve, the lipid kinase that converts PI(3)P to PI(3,5)P2 on LEL membranes, produced a similar phenotype in cells; therefore, we performed in vitro kinase assays and discovered that both SB203580 and SB202190 directly inhibited recombinant PIKfyve. Cancer cells treated with either drug likewise displayed significant reductions in the endogenous levels of PI(3,5)P2. Despite these results, SB203580-induced vacuolation was not entirely due to off-target inhibition of PIKfyve, as a drug-resistant p38α mutant suppressed vacuolation; and combined genetic deletion of both p38α and p38β dramatically sensitized cells to established PIKfyve inhibitors, including YM201636 and apilimod. The rate of vacuole dissolution (i.e., LEL fission), following the removal of apilimod, was also significantly reduced in cells treated with BIRB-796, a structurally unrelated p38 MAPK inhibitor. Thus, our studies indicate that pyridinyl imidazole p38 MAPK inhibitors induce cytoplasmic vacuolation through the combined inhibition of both PIKfyve and p38 MAPKs, and more generally, that p38 MAPKs act epistatically to PIKfyve, most likely to promote LEL fission.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Update of
-
Unexpected inhibition of the lipid kinase PIKfyve reveals an epistatic role for p38 MAPKs in endolysosomal fission and volume control.bioRxiv [Preprint]. 2023 Mar 14:2023.03.13.532495. doi: 10.1101/2023.03.13.532495. bioRxiv. 2023. Update in: Cell Death Dis. 2024 Jan 22;15(1):80. doi: 10.1038/s41419-024-06423-0 PMID: 36993747 Free PMC article. Updated. Preprint.
Similar articles
-
Unexpected inhibition of the lipid kinase PIKfyve reveals an epistatic role for p38 MAPKs in endolysosomal fission and volume control.bioRxiv [Preprint]. 2023 Mar 14:2023.03.13.532495. doi: 10.1101/2023.03.13.532495. bioRxiv. 2023. Update in: Cell Death Dis. 2024 Jan 22;15(1):80. doi: 10.1038/s41419-024-06423-0 PMID: 36993747 Free PMC article. Updated. Preprint.
-
Apilimod, a candidate anticancer therapeutic, arrests not only PtdIns(3,5)P2 but also PtdIns5P synthesis by PIKfyve and induces bafilomycin A1-reversible aberrant endomembrane dilation.PLoS One. 2018 Sep 21;13(9):e0204532. doi: 10.1371/journal.pone.0204532. eCollection 2018. PLoS One. 2018. PMID: 30240452 Free PMC article.
-
Snx10 and PIKfyve are required for lysosome formation in osteoclasts.J Cell Biochem. 2020 Apr;121(4):2927-2937. doi: 10.1002/jcb.29534. Epub 2019 Nov 6. J Cell Biochem. 2020. PMID: 31692073
-
PIKfyve: Partners, significance, debates and paradoxes.Cell Biol Int. 2008 Jun;32(6):591-604. doi: 10.1016/j.cellbi.2008.01.006. Epub 2008 Jan 25. Cell Biol Int. 2008. PMID: 18304842 Free PMC article. Review.
-
Small molecule PIKfyve inhibitors as cancer therapeutics: Translational promises and limitations.Toxicol Appl Pharmacol. 2019 Nov 15;383:114771. doi: 10.1016/j.taap.2019.114771. Epub 2019 Oct 16. Toxicol Appl Pharmacol. 2019. PMID: 31628917 Review.
Cited by
-
Inhibition of PIKfyve Leads to Lysosomal Disorders via Dysregulation of mTOR Signaling.Cells. 2024 May 30;13(11):953. doi: 10.3390/cells13110953. Cells. 2024. PMID: 38891085 Free PMC article.
-
Selective Termination of Autophagy-Dependent Cancers.Cells. 2024 Jun 25;13(13):1096. doi: 10.3390/cells13131096. Cells. 2024. PMID: 38994949 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

