A Mixture of T-Cell Epitope Peptides Derived from Human Respiratory Syncytial Virus F Protein Conferred Protection in DR1-TCR Tg Mice
- PMID: 38250890
- PMCID: PMC10820450
- DOI: 10.3390/vaccines12010077
A Mixture of T-Cell Epitope Peptides Derived from Human Respiratory Syncytial Virus F Protein Conferred Protection in DR1-TCR Tg Mice
Abstract
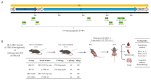
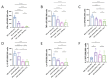
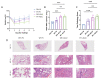
Human respiratory syncytial virus (HRSV) poses a significant disease burden on global health. To date, two vaccines that primarily induce humoral immunity to prevent HRSV infection have been approved, whereas vaccines that primarily induce T-cell immunity have not yet been well-represented. To address this gap, 25 predicted T-cell epitope peptides derived from the HRSV fusion protein with high human leukocyte antigen (HLA) binding potential were synthesized, and their ability to be recognized by PBMC from previously infected HRSV cases was assessed using an ELISpot assay. Finally, nine T-cell epitope peptides were selected, each of which was recognized by at least 20% of different donors' PBMC as potential vaccine candidates to prevent HRSV infection. The protective efficacy of F-9PV, a combination of nine peptides along with CpG-ODN and aluminum phosphate (Al) adjuvants, was validated in both HLA-humanized mice (DR1-TCR transgenic mice, Tg mice) and wild-type (WT) mice. The results show that F-9PV significantly enhanced protection against viral challenge as evidenced by reductions in viral load and pathological lesions in mice lungs. In addition, F-9PV elicits robust Th1-biased response, thereby mitigating the potential safety risk of Th2-induced respiratory disease during HRSV infection. Compared to WT mice, the F-9PV mice exhibited superior protection and immunogenicity in Tg mice, underscoring the specificity for human HLA. Overall, our results demonstrate that T-cell epitope peptides provide protection against HRSV infection in animal models even in the absence of neutralizing antibodies, indicating the feasibility of developing an HRSV T-cell epitope peptide-based vaccine.
Keywords: T-cell epitope; human respiratory syncytial virus; peptide vaccine.
Conflict of interest statement
We do not have conflicts of interest associated with this publication, and there has been no financial support for this work that could have influenced its outcome. This manuscript is original, has not been previously published, and is not currently under consideration by another journal.
Figures
Similar articles
-
Influence of Respiratory Syncytial Virus F Glycoprotein Conformation on Induction of Protective Immune Responses.J Virol. 2016 May 12;90(11):5485-5498. doi: 10.1128/JVI.00338-16. Print 2016 Jun 1. J Virol. 2016. PMID: 27009962 Free PMC article.
-
A single, low dose of a cGMP recombinant BCG vaccine elicits protective T cell immunity against the human respiratory syncytial virus infection and prevents lung pathology in mice.Vaccine. 2017 Feb 1;35(5):757-766. doi: 10.1016/j.vaccine.2016.12.048. Epub 2017 Jan 5. Vaccine. 2017. PMID: 28065474
-
Intranasal immunization with HRSV prefusion F protein and CpG adjuvant elicits robust protective effects in mice.Vaccine. 2022 Nov 8;40(47):6830-6838. doi: 10.1016/j.vaccine.2022.09.071. Epub 2022 Oct 15. Vaccine. 2022. PMID: 36253219
-
CD8+ T cell immunity against human respiratory syncytial virus.Vaccine. 2014 Oct 21;32(46):6130-7. doi: 10.1016/j.vaccine.2014.08.063. Epub 2014 Sep 16. Vaccine. 2014. PMID: 25223272 Review.
-
New Insights Contributing to the Development of Effective Vaccines and Therapies to Reduce the Pathology Caused by hRSV.Int J Mol Sci. 2017 Aug 11;18(8):1753. doi: 10.3390/ijms18081753. Int J Mol Sci. 2017. PMID: 28800119 Free PMC article. Review.
References
-
- Li Y., Wang X., Blau D.M., Caballero M.T., Feikin D.R., Gill C.J., Madhi S.A., Omer S.B., Simões E.A.F., Campbell H., et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: A systematic analysis. Lancet. 2022;399:2047–2064. doi: 10.1016/S0140-6736(22)00478-0. - DOI - PMC - PubMed
-
- Rijsbergen L.C., Lamers M.M., Comvalius A.D., Koutstaal R.W., Schipper D., Duprex W.P., Haagmans B.L., de Vries R.D., de Swart R.L. Human Respiratory Syncytial Virus Subgroup A and B Infections in Nasal, Bronchial, Small-Airway, and Organoid-Derived Respiratory Cultures. mSphere. 2021;6:e00237-21. doi: 10.1128/mSphere.00237-21. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

