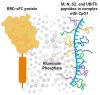
RBD-Protein/Peptide Vaccine UB-612 Elicits Mucosal and Fc-Mediated Antibody Responses against SARS-CoV-2 in Cynomolgus Macaques
- PMID: 38250853
- PMCID: PMC10818657
- DOI: 10.3390/vaccines12010040
RBD-Protein/Peptide Vaccine UB-612 Elicits Mucosal and Fc-Mediated Antibody Responses against SARS-CoV-2 in Cynomolgus Macaques
Abstract
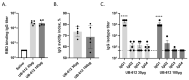
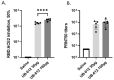
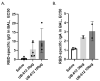
Antibodies provide critical protective immunity against COVID-19, and the Fc-mediated effector functions and mucosal antibodies also contribute to the protection. To expand the characterization of humoral immunity stimulated by subunit protein-peptide COVID-19 vaccine UB-612, preclinical studies in non-human primates were undertaken to investigate mucosal secretion and the effector functionality of vaccine-induced antibodies in antibody-dependent monocyte phagocytosis (ADMP) and antibody-dependent NK cell activation (ADNKA) assays. In cynomolgus macaques, UB-612 induced potent serum-neutralizing, RBD-specific IgG binding, ACE2 binding-inhibition antibodies, and antibodies with Fc-mediated effector functions in ADMP and ADNKA assays. Additionally, immunized animals developed mucosal antibodies in bronchoalveolar lavage fluids (BAL). The level of mucosal or serum ADMP and ADNKA antibodies was found to be UB-612 dose-dependent. Our results highlight that the novel subunit UB-612 vaccine is a potent B-cell immunogen inducing polyfunctional antibody responses contributing to anti-viral immunity and vaccine efficacy.
Keywords: ADCP; ADMP; ADNKA; COVID-19; Fc-mediated effector function; RBD; SARS-CoV-2; antibody; non-human primates; subunit; vaccine.
Conflict of interest statement
S.W., F.G., V.R., J.W., B.T., J.S., M.H., J.-C.D. and A.R. were employed by Vaxxinity, and all other authors declare no conflict of interest.
Figures
Similar articles
-
Serum Fc-Mediated Monocyte Phagocytosis Activity Is Stable for Several Months after SARS-CoV-2 Asymptomatic and Mildly Symptomatic Infection.Microbiol Spectr. 2022 Dec 21;10(6):e0183722. doi: 10.1128/spectrum.01837-22. Epub 2022 Nov 14. Microbiol Spectr. 2022. PMID: 36374040 Free PMC article.
-
A novel RBD-protein/peptide vaccine elicits broadly neutralizing antibodies and protects mice and macaques against SARS-CoV-2.Emerg Microbes Infect. 2022 Dec;11(1):2724-2734. doi: 10.1080/22221751.2022.2140608. Emerg Microbes Infect. 2022. PMID: 36287714 Free PMC article.
-
Evaluation of the humoral and mucosal immune response of a multiepitope vaccine against COVID-19 in pigs.Front Immunol. 2023 Dec 20;14:1276950. doi: 10.3389/fimmu.2023.1276950. eCollection 2023. Front Immunol. 2023. PMID: 38179057 Free PMC article.
-
Deciphering Fc-effector functions against SARS-CoV-2.Trends Microbiol. 2024 Aug;32(8):756-768. doi: 10.1016/j.tim.2024.01.005. Epub 2024 Feb 15. Trends Microbiol. 2024. PMID: 38365562 Review.
-
Difference in respiratory syncytial virus-specific Fc-mediated antibody effector functions between children and adults.Clin Exp Immunol. 2023 Dec 11;214(1):79-93. doi: 10.1093/cei/uxad101. Clin Exp Immunol. 2023. PMID: 37605554 Free PMC article. Review.
References
-
- World Health Organization WHO Coronavirus (COVID-19) Dashboard. 2023. [(accessed on 17 November 2023)]. Available online: https://covid19.who.int/
-
- Chen X., Chen Z., Azman A.S., Sun R., Lu W., Zheng N., Zhou J., Wu Q., Deng X., Zhao Z., et al. Neutralizing Antibodies Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Variants Induced by Natural Infection or Vaccination: A Systematic Review and Pooled Analysis. Clin. Infect. Dis. 2022;74:734–742. doi: 10.1093/cid/ciab646. - DOI - PMC - PubMed
-
- Kim Y.-I., Kim S.-M., Park S.-J., Kim E.-H., Yu K.-M., Chang J.-H., Kim E.J., Casel M.A.B., Rollon R., Jang S.-G., et al. Critical role of neutralizing antibody for SARS-CoV-2 reinfection and transmission. Emerg. Microbes Infect. 2021;10:152–160. doi: 10.1080/22221751.2021.1872352. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

