Peptide receptor radionuclide therapy combinations for neuroendocrine tumours in ongoing clinical trials: status 2023
- PMID: 38250038
- PMCID: PMC10797289
- DOI: 10.7150/thno.91268
Peptide receptor radionuclide therapy combinations for neuroendocrine tumours in ongoing clinical trials: status 2023
Abstract
A growing body of literature reports on the combined use of peptide receptor radionuclide therapy (PRRT) with other anti-tumuor therapies in order to anticipate synergistic effects with perhaps increased safety issues. Combination treatments to enhance PRRT outcome are based on improved tumour perfusion, upregulation of somatostatin receptors (SSTR), radiosensitization with DNA damaging agents or targeted therapies. Several Phase 1 or 2 trials are currently recruiting patients in combined regimens. The combination of PRRT with cytotoxic chemotherapy, capecitabine and temozolomide (CAPTEM), seems to become clinically useful especially in pancreatic neuroendocrine tumours (pNETs) with acceptable safety profile. Neoadjuvant PRRT prior to surgery, PRRT combinations of intravenous and intraarterial routes of application, combinations of PRRT with differently radiolabelled (alpha, beta, Auger) SSTR-targeting agonists and antagonists, inhibitors of immune checkpoints (ICIs), poly (ADP-ribose) polymerase-1 (PARP1i), tyrosine kinase (TKI), DNA-dependent protein kinase, ribonucleotide reductase or DNA methyltransferase (DMNT) are tested in currently ongoing clinical trials. The combination with [131I]I-MIBG in rare NETs (such as paraganglioma, pheochromocytoma) and new non-SSTR-targeting radioligands are used in the personalization process of treatment. The present review will provide an overview of the current status of ongoing PRRT combination treatments.
Keywords: PRRT; SSTR; chemotherapy; combination treatments; neuroendocrine tumours.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
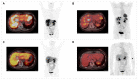
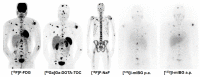
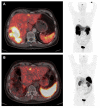
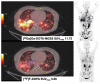
Similar articles
-
Combination treatments to enhance peptide receptor radionuclide therapy of neuroendocrine tumours.Eur J Nucl Med Mol Imaging. 2020 Apr;47(4):907-921. doi: 10.1007/s00259-019-04499-x. Epub 2019 Sep 6. Eur J Nucl Med Mol Imaging. 2020. PMID: 31492995 Review.
-
Capecitabine-Temozolomide in Advanced Grade 2 and Grade 3 Neuroendocrine Neoplasms: Benefits of Chemotherapy in Neuroendocrine Neoplasms with Significant 18FDG Uptake.Neuroendocrinology. 2021;111(10):998-1004. doi: 10.1159/000511987. Epub 2020 Oct 5. Neuroendocrinology. 2021. PMID: 33017827
-
Peptide Receptor Radionuclide Therapy for Pancreatic Neuroendocrine Tumours.Curr Radiopharm. 2019;12(2):126-134. doi: 10.2174/1874471012666190201164132. Curr Radiopharm. 2019. PMID: 30714538 Review.
-
Peptide Receptor Radionuclide Therapy for Advanced Gastroenteropancreatic Neuroendocrine Tumors - from oncology perspective.Nucl Med Rev Cent East Eur. 2018;21(2). doi: 10.5603/NMR.2018.0019. Nucl Med Rev Cent East Eur. 2018. PMID: 29741203 Review.
-
Peptide Receptor Radionuclide Therapy as a Novel Treatment for Metastatic and Invasive Phaeochromocytoma and Paraganglioma.Neuroendocrinology. 2019;109(4):287-298. doi: 10.1159/000499497. Epub 2019 Mar 12. Neuroendocrinology. 2019. PMID: 30856620 Review.
Cited by
-
Liver-Directed Locoregional Therapies for Neuroendocrine Liver Metastases: Recent Advances and Management.Curr Oncol. 2024 Apr 5;31(4):2076-2091. doi: 10.3390/curroncol31040154. Curr Oncol. 2024. PMID: 38668057 Free PMC article. Review.
-
Neuroendocrine Neoplasia: From Pathophysiology to Novel Therapeutic Approaches.Biomedicines. 2024 Apr 4;12(4):801. doi: 10.3390/biomedicines12040801. Biomedicines. 2024. PMID: 38672156 Free PMC article.
-
Advances in Radionuclide Therapies for Patients with Neuro-endocrine Tumors.Curr Oncol Rep. 2024 May;26(5):551-561. doi: 10.1007/s11912-024-01521-w. Epub 2024 Apr 10. Curr Oncol Rep. 2024. PMID: 38598035 Free PMC article. Review.
References
-
- Strosberg JR, Caplin ME, Kunz PL, Ruszniewski PB, Bodei L, Hendifar A. et al. NETTER-1 Investigators. 177Lu-Dotatate plus long-acting octreotide versus highdose long-acting octreotide in patients with midgut neuroendocrine tumours (NETTER-1): final overall survival and long-term safety results from an open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 2021; 22(12):1752-1763. Epub 2021 Nov 15. Erratum in: Lancet Oncol. 2022;23(2):e59. - PubMed
-
- Kwekkeboom DJ, Krenning EP, Lebtahi R, Komminoth P, Kos-Kudła B, de Herder WW, Plöckinger U; Mallorca Consensus Conference Participants; European Neuroendocrine Tumor Society. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: peptide receptor radionuclide therapy with radiolabeled somatostatin analogs. Neuroendocrinology. 2009;90(2):220–6. - PubMed
-
- Pavel M, O'Toole D, Costa F, Capdevila J, Gross D, Kianmanesh R. et al. ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology. 2016;103(2):172–85. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

