Alzheimer's disease and microorganisms: the non-coding RNAs crosstalk
- PMID: 38249527
- PMCID: PMC10796784
- DOI: 10.3389/fncel.2023.1256100
Alzheimer's disease and microorganisms: the non-coding RNAs crosstalk
Abstract
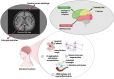
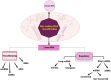
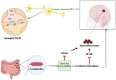
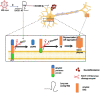
Alzheimer's disease (AD) is a complex, multifactorial disorder, influenced by a multitude of variables ranging from genetic factors, age, and head injuries to vascular diseases, infections, and various other environmental and demographic determinants. Among the environmental factors, the role of the microbiome in the genesis of neurodegenerative disorders (NDs) is gaining increased recognition. This paradigm shift is substantiated by an extensive body of scientific literature, which underscores the significant contributions of microorganisms, encompassing viruses and gut-derived bacteria, to the pathogenesis of AD. The mechanism by which microbial infection exerts its influence on AD hinges primarily on inflammation. Neuroinflammation, activated in response to microbial infections, acts as a defense mechanism for the brain but can inadvertently lead to unexpected neuropathological perturbations, ultimately contributing to NDs. Given the ongoing uncertainty surrounding the genetic factors underpinning ND, comprehensive investigations into environmental factors, particularly the microbiome and viral agents, are imperative. Recent advances in neuroscientific research have unveiled the pivotal role of non-coding RNAs (ncRNAs) in orchestrating various pathways integral to neurodegenerative pathologies. While the upstream regulators governing the pathological manifestations of microorganisms remain elusive, an in-depth exploration of the nuanced role of ncRNAs holds promise for the development of prospective therapeutic interventions. This review aims to elucidate the pivotal role of ncRNAs as master modulators in the realm of neurodegenerative conditions, with a specific focus on Alzheimer's disease.
Keywords: Alzheimer’s disease; gut microbiome; gut–brain axis; neurodegenerative disorders; non-coding RNAs; viral disease.
Copyright © 2024 Mohammadi-Pilehdarboni, Shenagari, Joukar, Naziri and Mansour-Ghanaei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Noncoding RNAs in Alzheimer's Disease: Overview of Functional and Therapeutic Significance.Curr Top Med Chem. 2024;24(19):1615-1634. doi: 10.2174/0115680266293212240405042540. Curr Top Med Chem. 2024. PMID: 38616763 Review.
-
Non-Coding RNA in Microglia Activation and Neuroinflammation in Alzheimer's Disease.J Inflamm Res. 2023 Sep 21;16:4165-4211. doi: 10.2147/JIR.S422114. eCollection 2023. J Inflamm Res. 2023. PMID: 37753266 Free PMC article. Review.
-
Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer's disease.Prog Neuropsychopharmacol Biol Psychiatry. 2021 Mar 2;106:110112. doi: 10.1016/j.pnpbp.2020.110112. Epub 2020 Sep 16. Prog Neuropsychopharmacol Biol Psychiatry. 2021. PMID: 32949638 Review.
-
Role of traditional Chinese medicine in ameliorating mitochondrial dysfunction via non-coding RNA signaling: Implication in the treatment of neurodegenerative diseases.Front Pharmacol. 2023 Mar 1;14:1123188. doi: 10.3389/fphar.2023.1123188. eCollection 2023. Front Pharmacol. 2023. PMID: 36937876 Free PMC article. Review.
-
Non-Coding RNAs as Novel Regulators of Neuroinflammation in Alzheimer's Disease.Front Immunol. 2022 Jun 2;13:908076. doi: 10.3389/fimmu.2022.908076. eCollection 2022. Front Immunol. 2022. PMID: 35720333 Free PMC article. Review.
References
-
- Alqahtani T., Deore S. L., Kide A. A., Shende B. A., Sharma R., Dadarao Chakole R., et al. . (2023). Mitochondrial dysfunction and oxidative stress in Alzheimer’s disease, and Parkinson’s disease, Huntington’s disease and amyotrophic lateral sclerosis-an updated review. Mitochondrion 71, 83–92. doi: 10.1016/j.mito.2023.05.007, PMID: - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

