Chronic Nicotine Consumption and Withdrawal Regulate Melanocortin Receptor, CRF, and CRF Receptor mRNA Levels in the Rat Brain
- PMID: 38248278
- PMCID: PMC10813117
- DOI: 10.3390/brainsci14010063
Chronic Nicotine Consumption and Withdrawal Regulate Melanocortin Receptor, CRF, and CRF Receptor mRNA Levels in the Rat Brain
Abstract
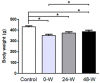
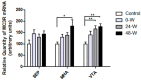
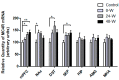
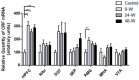
Alterations in the various neuropeptide systems in the mesocorticolimbic circuitry have been implicated in negative effects associated with drug withdrawal. The corticotropin-releasing factor (CRF) and α-melanocyte-stimulating hormone are two peptides that may be involved. This study investigated the regulatory effects of chronic nicotine exposure and withdrawal on the mRNA levels of melanocortin receptors (MC3R, MC4R), CRF, and CRF receptors (CRFR1 and CRFR2) expressed in the mesocorticolimbic system. Rats were given drinking water with nicotine or without nicotine (control group) for 12 weeks, after which they continued receiving nicotine (chronic exposure) or were withdrawn from nicotine for 24 or 48 h. The animals were decapitated following behavioral testing for withdrawal signs. Quantitative real-time PCR analysis demonstrated that nicotine exposure (with or without withdrawal) increased levels of CRF and CRFR1 mRNA in the amygdala, CRF mRNA in the medial prefrontal cortex, and CRFR1 mRNA in the septum. Nicotine withdrawal also enhanced MC3R and MC4R mRNA levels in different brain regions, while chronic nicotine exposure was associated with increased MC4R mRNA levels in the nucleus accumbens. These results suggest that chronic nicotine exposure and withdrawal regulate CRF and melanocortin signaling in the mesocorticolimbic system, possibly contributing to negative affective state and nicotine addiction.
Keywords: CRF; CRF1 receptor; MC3R; MC4R; mesocorticolimbic system; nicotine; withdrawal.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

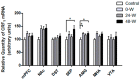
Similar articles
-
Chronic oral nicotine administration and withdrawal regulate the expression of neuropeptide Y and its receptors in the mesocorticolimbic system.Neuropeptides. 2021 Dec;90:102184. doi: 10.1016/j.npep.2021.102184. Epub 2021 Aug 17. Neuropeptides. 2021. PMID: 34425507
-
Gene expression of pro-opiomelanocortin and melanocortin receptors is regulated in the hypothalamus and mesocorticolimbic system following nicotine administration.Neurosci Lett. 2017 Jan 10;637:75-79. doi: 10.1016/j.neulet.2016.11.049. Epub 2016 Nov 24. Neurosci Lett. 2017. PMID: 27890744
-
Sustained AAV-mediated overexpression of CRF in the central amygdala diminishes the depressive-like state associated with nicotine withdrawal.Transl Psychiatry. 2014 Apr 22;4(4):e385. doi: 10.1038/tp.2014.25. Transl Psychiatry. 2014. PMID: 24755994 Free PMC article.
-
Neuropeptide systems and new treatments for nicotine addiction.Psychopharmacology (Berl). 2017 May;234(9-10):1419-1437. doi: 10.1007/s00213-016-4513-5. Epub 2016 Dec 28. Psychopharmacology (Berl). 2017. PMID: 28028605 Free PMC article. Review.
-
Actions of CRF and its analogs.Curr Med Chem. 1999 Nov;6(11):1035-53. Curr Med Chem. 1999. PMID: 10519912 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

