Extracellular Vesicles from a Biofilm of a Clinical Isolate of Candida albicans Negatively Impact on Klebsiella pneumoniae Adherence and Biofilm Formation
- PMID: 38247639
- PMCID: PMC10812662
- DOI: 10.3390/antibiotics13010080
Extracellular Vesicles from a Biofilm of a Clinical Isolate of Candida albicans Negatively Impact on Klebsiella pneumoniae Adherence and Biofilm Formation
Abstract
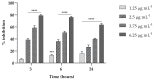
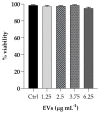
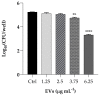
The opportunistic human fungal pathogen Candida albicans produces and releases into the surrounding medium extracellular vesicles (EVs), which are involved in some processes as communication between fungal cells and host-pathogen interactions during infection. Here, we have conducted the isolation of EVs produced by a clinical isolate of C. albicans during biofilm formation and proved their effect towards the ability of the Gram-negative bacterial pathogen Klebsiella pneumoniae to adhere to HaCaT cells and form a biofilm in vitro. The results represent the first evidence of an antagonistic action of fungal EVs against bacteria.
Keywords: Gram-negative bacteria; biofilm; candidiasis; extracellular vesicles; pathogenic fungi.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Candida albicans Biofilm-Derived Extracellular Vesicles Are Involved in the Tolerance to Caspofungin, Biofilm Detachment, and Fungal Proteolytic Activity.J Fungi (Basel). 2023 Nov 4;9(11):1078. doi: 10.3390/jof9111078. J Fungi (Basel). 2023. PMID: 37998883 Free PMC article.
-
Extracellular Vesicles Regulate Biofilm Formation and Yeast-to-Hypha Differentiation in Candida albicans.mBio. 2022 Jun 28;13(3):e0030122. doi: 10.1128/mbio.00301-22. Epub 2022 Apr 14. mBio. 2022. PMID: 35420476 Free PMC article.
-
Protein markers for Candida albicans EVs include claudin-like Sur7 family proteins.J Extracell Vesicles. 2020 Apr 16;9(1):1750810. doi: 10.1080/20013078.2020.1750810. eCollection 2020. J Extracell Vesicles. 2020. PMID: 32363014 Free PMC article.
-
Pathogenic factors in Candida biofilm-related infectious diseases.J Appl Microbiol. 2017 Feb;122(2):321-330. doi: 10.1111/jam.13330. Epub 2016 Nov 24. J Appl Microbiol. 2017. PMID: 27770500 Review.
-
Regulation of biofilm formation in Klebsiella pneumoniae.Front Microbiol. 2023 Sep 7;14:1238482. doi: 10.3389/fmicb.2023.1238482. eCollection 2023. Front Microbiol. 2023. PMID: 37744914 Free PMC article. Review.
Cited by
-
Evaluation of Potential Probiotic Properties and In Vivo Safety of Lactic Acid Bacteria and Yeast Strains Isolated from Traditional Home-Made Kefir.Foods. 2024 Mar 26;13(7):1013. doi: 10.3390/foods13071013. Foods. 2024. PMID: 38611319 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

