Characterization of Antibiotic Treatment among Children Aged 0-59 Months Hospitalized for Acute Bacterial Gastroenteritis in Israel
- PMID: 38247623
- PMCID: PMC10812600
- DOI: 10.3390/antibiotics13010064
Characterization of Antibiotic Treatment among Children Aged 0-59 Months Hospitalized for Acute Bacterial Gastroenteritis in Israel
Abstract
Background: We examined the extent and correlates of appropriate antibiotic use among children hospitalized with bacterial acute gastroenteritis (AGE) in Israel, a high-income country setting.
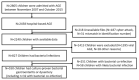
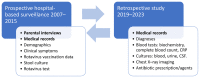
Methods: Data were collected from children aged 0-59 months who participated in active hospital-based surveillance of AGE undertaken during 2007-2015. Bacterial AGE was defined as having a positive stool culture for Salmonella, Shigella, Campylobacter, or dysentery. Appropriate antibiotic use was defined as the administration of ciprofloxacin, azithromycin, or third-generation cephalosporins during hospitalization or at discharge.
Results: Overall, 550 children had bacterial AGE; of those, 369 (67.1% [95% CI 63.1-70.9]) received antibiotics, mostly azithromycin (61.8%) and third-generation cephalosporins (37.9%). Appropriate antibiotic treatment was given to 318/550 (57.8% [95% CI 53.7-61.9]). Children aged 0-11 months vs. 24-49 months were more likely to receive appropriate antibiotic treatment (OR = 1.90 [95% CI 1.09-3.33]). Having dysentery (OR = 5.30 [95% CI 3.35-8.39]), performing blood culture (OR = 1.59 [95% CI 1.02-2.48]), and C-reactive protein (CRP) levels (OR = 1.01 [95% CI 1.01-1.02]) were positively associated with receiving appropriate antibiotic treatment.
Conclusions: Most children with bacterial AGE received appropriate antibiotic treatment, which correlated with young age, dysentery, CRP level, and performing blood culture, suggesting more severe illness, thus supporting the clinical decisions of physicians.
Keywords: antibiotics use; children; culture-proven bacterial gastroenteritis; dysentery; high-income country; hospitalization.
Conflict of interest statement
The authors report that there are no competing interests to declare.
Figures
Similar articles
-
Clinical dysentery in hospitalized children.Infection. 2002 Jun;30(3):132-5. doi: 10.1007/s15010-002-2166-2. Infection. 2002. PMID: 12120936
-
Campylobacter gastroenteritis in children in north-eastern Israel comparison with other common pathogens.Sci Rep. 2020 Apr 2;10(1):5823. doi: 10.1038/s41598-020-62744-y. Sci Rep. 2020. PMID: 32242070 Free PMC article.
-
Convulsions in children hospitalized for acute gastroenteritis.Sci Rep. 2021 Aug 5;11(1):15874. doi: 10.1038/s41598-021-95202-4. Sci Rep. 2021. PMID: 34354134 Free PMC article.
-
Enteropathogens and antibiotics.Enferm Infecc Microbiol Clin (Engl Ed). 2018 Jan;36(1):47-54. doi: 10.1016/j.eimc.2015.06.015. Epub 2015 Aug 12. Enferm Infecc Microbiol Clin (Engl Ed). 2018. PMID: 26277207 Review. English, Spanish.
-
Identification and management of Shigella infection in children with diarrhoea: a systematic review and meta-analysis.Lancet Glob Health. 2017 Dec;5(12):e1235-e1248. doi: 10.1016/S2214-109X(17)30392-3. Lancet Glob Health. 2017. PMID: 29132613 Free PMC article. Review.
Cited by
-
Factors associated with antibiotic use in children hospitalized for acute viral gastroenteritis and the relation to rotavirus vaccination.Hum Vaccin Immunother. 2024 Dec 31;20(1):2396707. doi: 10.1080/21645515.2024.2396707. Epub 2024 Sep 9. Hum Vaccin Immunother. 2024. PMID: 39248509 Free PMC article.
References
-
- Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S., Wu Y., Sow S.O., Sur D., Breiman R.F., et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. - DOI - PubMed
-
- Kotloff K.L., Nasrin D., Blackwelder W.C., Wu Y., Farag T., Panchalingham S., Sow S.O., Sur D., Zaidi A.K., Faruque A.S., et al. The incidence, aetiology, and adverse clinical consequences of less severe diarrhoeal episodes among infants and children residing in low-income and middle-income countries: A 12-month case-control study as a follow-on to the Global Enteric Multicenter Study. Lancet Glob. Health. 2019;7:e568–e584. doi: 10.1016/S2214-109X(19)30076-2. - DOI - PMC - PubMed
-
- Kotloff K.L., Platts-Mills J.A., Nasrin D., Roose A., Blackwelder W.C., Levine M.M. Global burden of diarrheal diseases among children in developing countries: Incidence, etiology, and insights from new molecular diagnostic techniques. Vaccine. 2017;35:6783–6789. doi: 10.1016/j.vaccine.2017.07.036. - DOI - PubMed
-
- Platts-Mills J.A., Liu J., Rogawski E.T., Kabir F., Lertsethtakarn P., Siguas M., Khan S.S., Praharaj I., Murei A., Nshama R., et al. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: A reanalysis of the MAL-ED cohort study. Lancet Glob. Health. 2018;6:e1309–e1318. doi: 10.1016/S2214-109X(18)30349-8. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

