A novel avian intestinal epithelial cell line: its characterization and exploration as an in vitro infection culture model for Eimeria species
- PMID: 38243250
- PMCID: PMC10799501
- DOI: 10.1186/s13071-023-06090-8
A novel avian intestinal epithelial cell line: its characterization and exploration as an in vitro infection culture model for Eimeria species
Abstract
Background: The gastrointestinal epithelium plays an important role in directing recognition by the immune system, and epithelial cells provide the host's front line of defense against microorganisms. However, it is difficult to cultivate avian intestinal epithelial cells in vitro for lengthy periods, and the lack of available cell lines limits the research on avian intestinal diseases and nutritional regulation. Chicken coccidiosis is a serious intestinal disease that causes significant economic losses in the poultry industry. In vitro, some cell line models are beneficial for the development of Eimeria species; however, only partial reproduction can be achieved. Therefore, we sought to develop a new model with both the natural host and epithelial cell phenotypes.
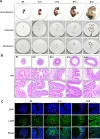
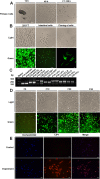
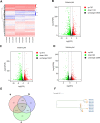
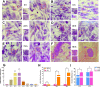
Methods: In this study, we use the SV40 large T antigen (SV40T) gene to generate an immortalized cell line. Single-cell screening technology was used to sort positive cell clusters with epithelial characteristics for passage. Polymerase chain reaction (PCR) identification, immunofluorescence detection, and bulk RNA sequencing analysis and validation were used to check the expression of epithelial cell markers and characterize the avian intestinal epithelial cell line (AIEC). AIECs were infected with sporozoites, and their ability to support the in vitro endogenous development of Eimeria tenella was assessed.
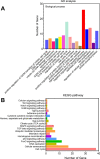
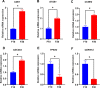
Results: This novel AIEC consistently expressed intestinal epithelial markers. Transcriptome assays revealed the upregulation of genes associated with proliferation and downregulation of genes associated with apoptosis. We sought to compare E. tenella infection between an existing fibroblast cell line (DF-1) and several passages of AIEC and found that the invasion efficiency was significantly increased relative to that of chicken fibroblast cell lines.
Conclusions: An AIEC will serve as a better in vitro research model, especially in the study of Eimeria species development and the mechanisms of parasite-host interactions. Using AIEC helps us understand the involvement of intestinal epithelial cells in the digestive tract and the immune defense of the chickens, which will contribute to the epithelial innate defense against microbial infection in the gastrointestinal tract.
Keywords: Avian embryo; Avian intestinal epithelial cell line (AIEC); Culture model; E. tenella.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Establishment of an in vitro chicken epithelial cell line model to investigate Eimeria tenella gamete development.Parasit Vectors. 2018 Jan 18;11(1):44. doi: 10.1186/s13071-018-2622-1. Parasit Vectors. 2018. PMID: 29347990 Free PMC article.
-
In vitro infection of Madin-Darby bovine kidney (MDBK) cells with Eimeria acervulina sporozoites: quantitative analysis of parasite cellular invasion and replication using real-time polymerase chain reaction (PCR).Parasitol Res. 2021 Jul;120(7):2689-2693. doi: 10.1007/s00436-021-07211-x. Epub 2021 Jun 19. Parasitol Res. 2021. PMID: 34146126 Free PMC article.
-
Eimeria tenella Eimeria-specific protein that interacts with apical membrane antigen 1 (EtAMA1) is involved in host cell invasion.Parasit Vectors. 2020 Jul 25;13(1):373. doi: 10.1186/s13071-020-04229-5. Parasit Vectors. 2020. PMID: 32711572 Free PMC article.
-
Intestinal immune responses to coccidiosis.Dev Comp Immunol. 2000 Mar-Apr;24(2-3):303-24. doi: 10.1016/s0145-305x(99)00080-4. Dev Comp Immunol. 2000. PMID: 10717295 Review.
-
Overview of Poultry Eimeria Life Cycle and Host-Parasite Interactions.Front Vet Sci. 2020 Jul 3;7:384. doi: 10.3389/fvets.2020.00384. eCollection 2020. Front Vet Sci. 2020. PMID: 32714951 Free PMC article. Review.
References
MeSH terms
Grants and funding
- 2023B04J0137/Science and technology project of Guangzhou
- 202110TD/Special fund for scientific innovation strategy-construction of high level Academy of Agriculture Science
- 202122TD/Special fund for scientific innovation strategy-construction of high level Academy of Agriculture Science
- R2020PY-JG013/Special fund for scientific innovation strategy-construction of high level Academy of Agriculture Science
- R2023PY-JG018/Special fund for scientific innovation strategy-construction of high level Academy of Agriculture Science
- XTXM202202/the Project of Collaborative Innovation Center of GDAAS
- 2022SDZG02/the open competition program of top ten critical priorities of Agricultural Science and Technology Innovation for the 14th Five-Year Plan of Guangdong Province
- 2021B1212050021/Science and Technology Plan Projects of Guangdong Province
- 2022KJ119/Guangdong Provincial special fund for modern Agriculture Industry Technology Innovation teams
LinkOut - more resources
Full Text Sources
Miscellaneous

