High UV damage and low repair, but not cytosine deamination, stimulate mutation hotspots at ETS binding sites in melanoma
- PMID: 38241433
- PMCID: PMC10823218
- DOI: 10.1073/pnas.2310854121
High UV damage and low repair, but not cytosine deamination, stimulate mutation hotspots at ETS binding sites in melanoma
Abstract
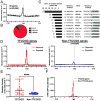
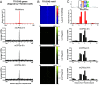
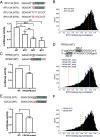
Noncoding mutation hotspots have been identified in melanoma and many of them occur at the binding sites of E26 transformation-specific (ETS) proteins; however, their formation mechanism and functional impacts are not fully understood. Here, we used UV (Ultraviolet) damage sequencing data and analyzed cyclobutane pyrimidine dimer (CPD) formation, DNA repair, and CPD deamination in human cells at single-nucleotide resolution. Our data show prominent CPD hotspots immediately after UV irradiation at ETS binding sites, particularly at sites with a conserved TTCCGG motif, which correlate with mutation hotspots identified in cutaneous melanoma. Additionally, CPDs are repaired slower at ETS binding sites than in flanking DNA. Cytosine deamination in CPDs to uracil is suggested as an important step for UV mutagenesis. However, we found that CPD deamination is significantly suppressed at ETS binding sites, particularly for the CPD hotspot on the 5' side of the ETS motif, arguing against a role for CPD deamination in promoting ETS-associated UV mutations. Finally, we analyzed a subset of frequently mutated promoters, including the ribosomal protein genes RPL13A and RPS20, and found that mutations in the ETS motif can significantly reduce the promoter activity. Thus, our data identify high UV damage and low repair, but not CPD deamination, as the main mechanism for ETS-associated mutations in melanoma and uncover important roles of often-overlooked mutation hotspots in perturbing gene transcription.
Keywords: CPD-seq 2.0; ETS; NER; mutagenesis.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Interventions to reduce harm from continued tobacco use.Cochrane Database Syst Rev. 2016 Oct 13;10(10):CD005231. doi: 10.1002/14651858.CD005231.pub3. Cochrane Database Syst Rev. 2016. PMID: 27734465 Free PMC article. Review.
-
Can a Liquid Biopsy Detect Circulating Tumor DNA With Low-passage Whole-genome Sequencing in Patients With a Sarcoma? A Pilot Evaluation.Clin Orthop Relat Res. 2025 Jan 1;483(1):39-48. doi: 10.1097/CORR.0000000000003161. Epub 2024 Jun 21. Clin Orthop Relat Res. 2025. PMID: 38905450
-
Evaluating Different Types of Cancer Survivorship Care [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Jul. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2019 Jul. PMID: 39602557 Free Books & Documents. Review.
Cited by
-
Nucleotide excision repair of aflatoxin-induced DNA damage within the 3D human genome organization.Nucleic Acids Res. 2024 Oct 28;52(19):11704-11719. doi: 10.1093/nar/gkae755. Nucleic Acids Res. 2024. PMID: 39258558 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

