Allosteric modulation of G protein-coupled receptors as a novel therapeutic strategy in neuropathic pain
- PMID: 38239234
- PMCID: PMC10792987
- DOI: 10.1016/j.apsb.2023.07.020
Allosteric modulation of G protein-coupled receptors as a novel therapeutic strategy in neuropathic pain
Abstract
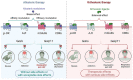
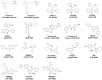
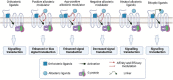
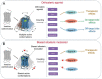
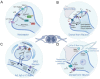
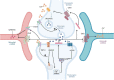
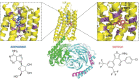
Neuropathic pain is a debilitating pathological condition that presents significant therapeutic challenges in clinical practice. Unfortunately, current pharmacological treatments for neuropathic pain lack clinical efficacy and often lead to harmful adverse reactions. As G protein-coupled receptors (GPCRs) are widely distributed throughout the body, including the pain transmission pathway and descending inhibition pathway, the development of novel neuropathic pain treatments based on GPCRs allosteric modulation theory is gaining momentum. Extensive research has shown that allosteric modulators targeting GPCRs on the pain pathway can effectively alleviate symptoms of neuropathic pain while reducing or eliminating adverse effects. This review aims to provide a comprehensive summary of the progress made in GPCRs allosteric modulators in the treatment of neuropathic pain, and discuss the potential benefits and adverse factors of this treatment. We will also concentrate on the development of biased agonists of GPCRs, and based on important examples of biased agonist development in recent years, we will describe universal strategies for designing structure-based biased agonists. It is foreseeable that, with the continuous improvement of GPCRs allosteric modulation and biased agonist theory, effective GPCRs allosteric drugs will eventually be available for the treatment of neuropathic pain with acceptable safety.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Cited by
-
The allosteric mechanism of mTOR activation can inform bitopic inhibitor optimization.Chem Sci. 2023 Dec 7;15(3):1003-1017. doi: 10.1039/d3sc04690g. eCollection 2024 Jan 17. Chem Sci. 2023. PMID: 38239681 Free PMC article.
-
G protein-coupled receptors (GPCRs): advances in structures, mechanisms, and drug discovery.Signal Transduct Target Ther. 2024 Apr 10;9(1):88. doi: 10.1038/s41392-024-01803-6. Signal Transduct Target Ther. 2024. PMID: 38594257 Free PMC article. Review.
-
Cannabinoids: Potential for Modulation and Enhancement When Combined with Vitamin B12 in Case of Neurodegenerative Disorders.Pharmaceuticals (Basel). 2024 Jun 20;17(6):813. doi: 10.3390/ph17060813. Pharmaceuticals (Basel). 2024. PMID: 38931480 Free PMC article. Review.
References
-
- IASP-Pain.org [homepage on the Internet]. Washington: International Association for th-e Study of Pain; [updated 2021; cited 2022 June 9]. Available from: http://www.iasp-pain.org/Education/Content.aspx? ItemNumber=1698&navItem....
-
- Attal N., Lanteri-Minet M., Laurent B., Fermanian J., Bouhassira D. The specific disease burden of neuropathic pain: results of a French nationwide survey. Pain. 2011;152:2836–2843. - PubMed
-
- Doth A.H., Hansson P.T., Jensen M.P., Taylor R.S. The burden of neuropathic pain: a systematic review and meta-analysis of health utilities. Pain. 2010;149:338–344. - PubMed
-
- van Hecke O., Austin S.K., Khan R.A., Smith B.H., Torrance N. Neuropathic pain in the general population: a systematic review of epidemiological studies. Pain. 2014;155:654–662. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

