Benzosceptrin C induces lysosomal degradation of PD-L1 and promotes antitumor immunity by targeting DHHC3
- PMID: 38237597
- PMCID: PMC10897506
- DOI: 10.1016/j.xcrm.2023.101357
Benzosceptrin C induces lysosomal degradation of PD-L1 and promotes antitumor immunity by targeting DHHC3
Abstract
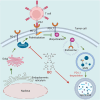
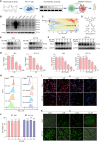
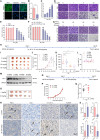
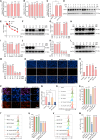
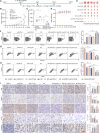
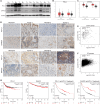
Programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) blockade has become a mainstay of cancer immunotherapy. Targeting the PD-1/PD-L1 axis with small molecules is an attractive approach to enhance antitumor immunity. Here, we identified a natural marine product, benzosceptrin C (BC), that enhances the cytotoxicity of T cells to cancer cells by reducing the abundance of PD-L1. Furthermore, BC exerts its antitumor effect in mice bearing MC38 tumors by activating tumor-infiltrating T cell immunity. Mechanistic studies suggest that BC can prevent palmitoylation of PD-L1 by inhibiting DHHC3 enzymatic activity. Subsequently, PD-L1 is transferred from the membrane to the cytoplasm and cannot return to the membrane via recycling endosomes, triggering lysosome-mediated degradation of PD-L1. Moreover, the combination of BC and anti-CTLA4 effectively enhances antitumor T cell immunity. Our findings reveal a previously unrecognized antitumor mechanism of BC and represent an alternative immune checkpoint blockade (ICB) therapeutic strategy to enhance the efficacy of cancer immunotherapy.
Keywords: DHHC3; PD-L1; benzosceptrin C; cancer immunotherapy; colorectal cancer.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests All authors declare that they have no competing interests.
Figures


Similar articles
-
Tubeimoside-1 induces TFEB-dependent lysosomal degradation of PD-L1 and promotes antitumor immunity by targeting mTOR.Acta Pharm Sin B. 2021 Oct;11(10):3134-3149. doi: 10.1016/j.apsb.2021.03.039. Epub 2021 Apr 1. Acta Pharm Sin B. 2021. PMID: 34745852 Free PMC article.
-
Inhibiting PD-L1 palmitoylation enhances T-cell immune responses against tumours.Nat Biomed Eng. 2019 Apr;3(4):306-317. doi: 10.1038/s41551-019-0375-6. Epub 2019 Mar 25. Nat Biomed Eng. 2019. PMID: 30952982
-
Treating ICB-resistant cancer by inhibiting PD-L1 via DHHC3 degradation induced by cell penetrating peptide-induced chimera conjugates.Cell Death Dis. 2024 Sep 30;15(9):701. doi: 10.1038/s41419-024-07073-y. Cell Death Dis. 2024. PMID: 39349454 Free PMC article.
-
PD-L1 degradation pathway and immunotherapy for cancer.Cell Death Dis. 2020 Nov 6;11(11):955. doi: 10.1038/s41419-020-03140-2. Cell Death Dis. 2020. PMID: 33159034 Free PMC article. Review.
-
PD-1/PD-L1 Blockade Therapy in Advanced Non-Small-Cell Lung Cancer: Current Status and Future Directions.Oncologist. 2019 Feb;24(Suppl 1):S31-S41. doi: 10.1634/theoncologist.2019-IO-S1-s05. Oncologist. 2019. PMID: 30819829 Free PMC article. Review.
Cited by
-
Posttranslational regulatory mechanism of PD-L1 in cancers and associated opportunities for novel small-molecule therapeutics.Acta Biochim Biophys Sin (Shanghai). 2024 May 31;56(10):1415-1424. doi: 10.3724/abbs.2024085. Acta Biochim Biophys Sin (Shanghai). 2024. PMID: 38826132 Free PMC article. Review.
-
5,7,4'-Trimethoxyflavone triggers cancer cell PD-L1 ubiquitin-proteasome degradation and facilitates antitumor immunity by targeting HRD1.MedComm (2020). 2024 Jun 27;5(7):e611. doi: 10.1002/mco2.611. eCollection 2024 Jul. MedComm (2020). 2024. PMID: 38938284 Free PMC article.
-
Development of small molecule drugs targeting immune checkpoints.Cancer Biol Med. 2024 May 9;21(5):382-99. doi: 10.20892/j.issn.2095-3941.2024.0034. Cancer Biol Med. 2024. PMID: 38727005 Free PMC article. Review.
References
-
- Peters S., Reck M., Smit E.F., Mok T., Hellmann M.D. How to make the best use of immunotherapy as first-line treatment of advanced/metastatic non-small-cell lung cancer. Ann. Oncol. 2019;30:884–896. - PubMed
-
- Fan X., Jin J., Yan L., Liu L., Li Q., Xu Y. The impaired anti-tumoral effect of immune surveillance cells in the immune microenvironment of gastric cancer. Clin. Immunol. 2020;219 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

