The roles of tissue resident macrophages in health and cancer
- PMID: 38229178
- PMCID: PMC10790434
- DOI: 10.1186/s40164-023-00469-0
The roles of tissue resident macrophages in health and cancer
Abstract
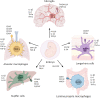
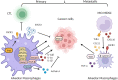
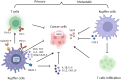
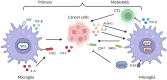
As integral components of the immune microenvironment, tissue resident macrophages (TRMs) represent a self-renewing and long-lived cell population that plays crucial roles in maintaining homeostasis, promoting tissue remodeling after damage, defending against inflammation and even orchestrating cancer progression. However, the exact functions and roles of TRMs in cancer are not yet well understood. TRMs exhibit either pro-tumorigenic or anti-tumorigenic effects by engaging in phagocytosis and secreting diverse cytokines, chemokines, and growth factors to modulate the adaptive immune system. The life-span, turnover kinetics and monocyte replenishment of TRMs vary among different organs, adding to the complexity and controversial findings in TRMs studies. Considering the complexity of tissue associated macrophage origin, macrophages targeting strategy of each ontogeny should be carefully evaluated. Consequently, acquiring a comprehensive understanding of TRMs' origin, function, homeostasis, characteristics, and their roles in cancer for each specific organ holds significant research value. In this review, we aim to provide an outline of homeostasis and characteristics of resident macrophages in the lung, liver, brain, skin and intestinal, as well as their roles in modulating primary and metastatic cancer, which may inform and serve the future design of targeted therapies.
Keywords: Bone-marrow derived macrophages; Cancer; Homeostasis; Monocytes; Tissue resident macrophages.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Function of alveolar macrophages in lung cancer microenvironment.Inflamm Regen. 2024 May 8;44(1):23. doi: 10.1186/s41232-024-00335-4. Inflamm Regen. 2024. PMID: 38720352 Free PMC article. Review.
-
Tissue-Resident Macrophages in Cancer: Friend or Foe?Cancer Med. 2024 Nov;13(21):e70387. doi: 10.1002/cam4.70387. Cancer Med. 2024. PMID: 39494816 Free PMC article. Review.
-
Fibrosis induced by resident macrophages has divergent roles in pancreas inflammatory injury and PDAC.Nat Immunol. 2023 Sep;24(9):1443-1457. doi: 10.1038/s41590-023-01579-x. Epub 2023 Aug 10. Nat Immunol. 2023. PMID: 37563309 Free PMC article.
-
Tissue-Resident Macrophages in Solid Organ Transplantation: Harmful or Protective?J Immunol. 2024 Apr 1;212(7):1051-1061. doi: 10.4049/jimmunol.2300625. J Immunol. 2024. PMID: 38498808 Review.
-
Understanding tissue-resident macrophages unlocks the potential for novel combinatorial strategies in breast cancer.Front Immunol. 2024 Jul 22;15:1375528. doi: 10.3389/fimmu.2024.1375528. eCollection 2024. Front Immunol. 2024. PMID: 39104525 Free PMC article. Review.
Cited by
-
Function of alveolar macrophages in lung cancer microenvironment.Inflamm Regen. 2024 May 8;44(1):23. doi: 10.1186/s41232-024-00335-4. Inflamm Regen. 2024. PMID: 38720352 Free PMC article. Review.
-
Methylation and transcriptomic profiling reveals short term and long term regulatory responses in polarized macrophages.Comput Struct Biotechnol J. 2024 Aug 17;25:143-152. doi: 10.1016/j.csbj.2024.08.018. eCollection 2024 Dec. Comput Struct Biotechnol J. 2024. PMID: 39257962 Free PMC article.
-
Immunosuppressive tumor microenvironment in the progression, metastasis, and therapy of hepatocellular carcinoma: from bench to bedside.Exp Hematol Oncol. 2024 Aug 1;13(1):72. doi: 10.1186/s40164-024-00539-x. Exp Hematol Oncol. 2024. PMID: 39085965 Free PMC article. Review.
-
Macrophage Functions in Psoriasis: Lessons from Mouse Models.Int J Mol Sci. 2024 May 13;25(10):5306. doi: 10.3390/ijms25105306. Int J Mol Sci. 2024. PMID: 38791342 Free PMC article. Review.
-
Tissue-Resident Macrophages in Cancer: Friend or Foe?Cancer Med. 2024 Nov;13(21):e70387. doi: 10.1002/cam4.70387. Cancer Med. 2024. PMID: 39494816 Free PMC article. Review.
References
Publication types
Grants and funding
- 2023-803/Sichuan Provincial Cadre Health Research Project
- 2023NSFSC0703/Sichuan Science and technology Foundation Project
- 2023YFH0079/Sichuan Science and technology Foundation Project
- 2023NSFSC0720/Sichuan Science and technology Foundation Project
- TB2022090/Sichuan Special Funding for Postdoctoral Research Projects
LinkOut - more resources
Full Text Sources
Research Materials

