The adhesion GPCR GPR116/ADGRF5 has a dual function in pancreatic islets regulating somatostatin release and islet development
- PMID: 38228886
- PMCID: PMC10791652
- DOI: 10.1038/s42003-024-05783-9
The adhesion GPCR GPR116/ADGRF5 has a dual function in pancreatic islets regulating somatostatin release and islet development
Abstract
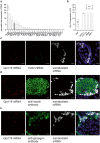
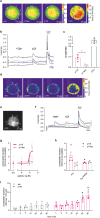
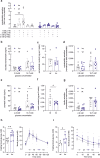
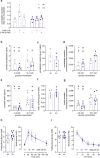
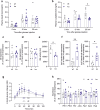
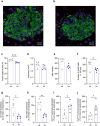
Glucose homeostasis is maintained by hormones secreted from different cell types of the pancreatic islets and controlled by manifold input including signals mediated through G protein-coupled receptors (GPCRs). RNA-seq analyses revealed expression of numerous GPCRs in mouse and human pancreatic islets, among them Gpr116/Adgrf5. GPR116 is an adhesion GPCR mainly found in lung and required for surfactant secretion. Here, we demonstrate that GPR116 is involved in the somatostatin release from pancreatic delta cells using a whole-body as well as a cell-specific knock-out mouse model. Interestingly, the whole-body GPR116 deficiency causes further changes such as decreased beta-cell mass, lower number of small islets, and reduced pancreatic insulin content. Glucose homeostasis in global GPR116-deficient mice is maintained by counter-acting mechanisms modulating insulin degradation. Our data highlight an important function of GPR116 in controlling glucose homeostasis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare the following competing interests: M.G.L. is employed by and shareholder of Novartis Pharma AG. All other authors declare no competing interests.
Figures
Similar articles
-
Regulation of pulmonary surfactant by the adhesion GPCR GPR116/ADGRF5 requires a tethered agonist-mediated activation mechanism.Elife. 2022 Sep 8;11:e69061. doi: 10.7554/eLife.69061. Elife. 2022. PMID: 36073784 Free PMC article.
-
Adhesion-GPCR Gpr116 (ADGRF5) expression inhibits renal acid secretion.Proc Natl Acad Sci U S A. 2020 Oct 20;117(42):26470-26481. doi: 10.1073/pnas.2007620117. Epub 2020 Oct 1. Proc Natl Acad Sci U S A. 2020. PMID: 33004624 Free PMC article.
-
G protein-coupled receptors as regulators of pancreatic islet functionality.Biochim Biophys Acta Mol Cell Res. 2022 May;1869(5):119235. doi: 10.1016/j.bbamcr.2022.119235. Epub 2022 Feb 10. Biochim Biophys Acta Mol Cell Res. 2022. PMID: 35151663 Review.
-
Orphan GPR116 mediates the insulin sensitizing effects of the hepatokine FNDC4 in adipose tissue.Nat Commun. 2021 May 20;12(1):2999. doi: 10.1038/s41467-021-22579-1. Nat Commun. 2021. PMID: 34016966 Free PMC article.
-
An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans.Pharmacol Ther. 2013 Sep;139(3):359-91. doi: 10.1016/j.pharmthera.2013.05.004. Epub 2013 May 18. Pharmacol Ther. 2013. PMID: 23694765 Review.
Cited by
-
The adhesion-GPCR ADGRF5 fuels breast cancer progression by suppressing the MMP8-mediated antitumorigenic effects.Cell Death Dis. 2024 Jun 27;15(6):455. doi: 10.1038/s41419-024-06855-8. Cell Death Dis. 2024. PMID: 38937435 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

