Susceptibility to acute cognitive dysfunction in aged mice is underpinned by reduced white matter integrity and microgliosis
- PMID: 38228820
- PMCID: PMC10791665
- DOI: 10.1038/s42003-023-05662-9
Susceptibility to acute cognitive dysfunction in aged mice is underpinned by reduced white matter integrity and microgliosis
Abstract
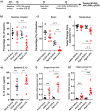
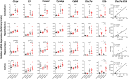
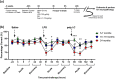
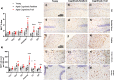
Age is a significant but heterogeneous risk factor for acute neuropsychiatric disturbances such as delirium. Neuroinflammation increases with aging but the determinants of underlying risk for acute dysfunction upon systemic inflammation are not clear. We hypothesised that, with advancing age, mice would become progressively more vulnerable to acute cognitive dysfunction and that neuroinflammation and neuronal integrity might predict heterogeneity in such vulnerability. Here we show region-dependent differential expression of microglial transcripts, but a ubiquitously observed primed signature: chronic Clec7a expression and exaggerated Il1b responses to systemic bacterial LPS. Cognitive frailty (vulnerability to acute disruption under acute stressors LPS and double stranded RNA; poly I:C) was increased in aged animals but showed heterogeneity and was significantly correlated with reduced myelin density, synaptic loss and severity of white matter microgliosis. The data indicate that white matter disruption and neuroinflammation may be key substrates of the progressive but heterogeneous risk for delirium in aged individuals.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Acute Inflammation Alters Brain Energy Metabolism in Mice and Humans: Role in Suppressed Spontaneous Activity, Impaired Cognition, and Delirium.J Neurosci. 2020 Jul 15;40(29):5681-5696. doi: 10.1523/JNEUROSCI.2876-19.2020. Epub 2020 Jun 8. J Neurosci. 2020. PMID: 32513828 Free PMC article.
-
Worsening cognitive impairment and neurodegenerative pathology progressively increase risk for delirium.Am J Geriatr Psychiatry. 2015 Apr;23(4):403-415. doi: 10.1016/j.jagp.2014.08.005. Epub 2014 Aug 15. Am J Geriatr Psychiatry. 2015. PMID: 25239680 Free PMC article.
-
T Cells Actively Infiltrate the White Matter of the Aging Monkey Brain in Relation to Increased Microglial Reactivity and Cognitive Decline.Front Immunol. 2021 Feb 16;12:607691. doi: 10.3389/fimmu.2021.607691. eCollection 2021. Front Immunol. 2021. PMID: 33664743 Free PMC article.
-
Delirium and cognitive decline: more than a coincidence.Curr Opin Neurol. 2013 Dec;26(6):634-9. doi: 10.1097/WCO.0000000000000030. Curr Opin Neurol. 2013. PMID: 24152819 Review.
-
Microglial Interferon Signaling and White Matter.Neurochem Res. 2017 Sep;42(9):2625-2638. doi: 10.1007/s11064-017-2307-8. Epub 2017 May 25. Neurochem Res. 2017. PMID: 28540600 Free PMC article. Review.
Cited by
-
Effect of JM-20 on Age-Related Cognitive Impairment in Mice.Neurochem Res. 2024 Nov 15;50(1):8. doi: 10.1007/s11064-024-04254-1. Neurochem Res. 2024. PMID: 39546064
-
Role of glia in delirium: proposed mechanisms and translational implications.Mol Psychiatry. 2024 Oct 27. doi: 10.1038/s41380-024-02801-4. Online ahead of print. Mol Psychiatry. 2024. PMID: 39463449 Review.
-
Discovery of novel protective agents for infection-related delirium through bispectral electroencephalography.Transl Psychiatry. 2024 Oct 3;14(1):413. doi: 10.1038/s41398-024-03130-4. Transl Psychiatry. 2024. PMID: 39358319 Free PMC article.
References
-
- Kirkwood, T. B. L. Understanding the odd science of aging. Cell 120 437–447 (2005). - PubMed
-
- Chesky, J. A. THE BIOLOGY OF AGING: OBSERVATIONS & PRINCIPLES (3rd Edition) By Robert Arking. Educational Gerontology33, 3-25 (2007).
-
- Esiri, M. M. Ageing and the brain. J. Pathol. 211, 181–187 (2007) 10.1002/path.2089. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

