Allogeneic Stem Cell Therapy for Acute Ischemic Stroke: The Phase 2/3 TREASURE Randomized Clinical Trial
- PMID: 38227308
- PMCID: PMC10792497
- DOI: 10.1001/jamaneurol.2023.5200
Allogeneic Stem Cell Therapy for Acute Ischemic Stroke: The Phase 2/3 TREASURE Randomized Clinical Trial
Abstract
Importance: Cell therapy is a promising treatment approach for stroke and other diseases. However, it is unknown whether MultiStem (HLCM051), a bone marrow-derived, allogeneic, multipotent adult progenitor cell product, has the potential to treat ischemic stroke.
Objective: To assess the efficacy and safety of MultiStem when administered within 18 to 36 hours of ischemic stroke onset.
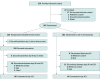
Design, setting, and participants: The Treatment Evaluation of Acute Stroke Using Regenerative Cells (TREASURE) multicenter, double-blind, parallel-group, placebo-controlled phase 2/3 randomized clinical trial was conducted at 44 academic and clinical centers in Japan between November 15, 2017, and March 29, 2022. Inclusion criteria were age 20 years or older, presence of acute ischemic stroke (National Institutes of Health Stroke Scale [NIHSS] score of 8-20 at baseline), confirmed acute infarction involving the cerebral cortex and measuring more than 2 cm on the major axis (determined with diffusion-weighted magnetic resonance imaging), and a modified Rankin Scale (mRS) score of 0 or 1 before stroke onset. Data analysis was performed between May 9 and August 15, 2022.
Exposure: Patients were randomly assigned to either intravenous MultiStem in 1 single unit of 1.2 billion cells or intravenous placebo within 18 to 36 hours of ischemic stroke onset.
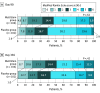
Main outcomes and measures: The primary end points were safety and excellent outcome at day 90, measured as a composite of a modified Rankin Scale (mRS) score of 1 or less, a NIHSS score of 1 or less, and a Barthel index score of 95 or greater. The secondary end points were excellent outcome at day 365, mRS score distribution at days 90 and 365, and mRS score of 0 to 1 and 0 to 2 at day 90. Statistical analysis of efficacy was performed using the Cochran-Mantel-Haenszel test.
Results: This study included 206 patients (104 received MultiStem and 102 received placebo). Their mean age was 76.5 (range, 35-95) years, and more than half of patients were men (112 [54.4%]). There were no between-group differences in primary and secondary end points. The proportion of excellent outcomes at day 90 did not differ significantly between the MultiStem and placebo groups (12 [11.5%] vs 10 [9.8%], P = .90; adjusted risk difference, 0.5% [95% CI, -7.3% to 8.3%]). The frequency of adverse events was similar between treatment groups.
Conclusions and relevance: In this randomized clinical trial, intravenous administration of allogeneic cell therapy within 18 to 36 hours of ischemic stroke onset was safe but did not improve short-term outcomes. Further research is needed to determine whether MultiStem therapy for ischemic stroke has a beneficial effect in patients who meet specific criteria, as indicated by the exploratory analyses in this study.
Trial registration: ClinicalTrials.gov Identifier: NCT02961504.
Conflict of interest statement
Figures

Similar articles
-
Treatment evaluation of acute stroke for using in regenerative cell elements (TREASURE) trial: Rationale and design.Int J Stroke. 2018 Jun;13(4):444-448. doi: 10.1177/1747493017743057. Epub 2017 Nov 14. Int J Stroke. 2018. PMID: 29134924
-
Safety and efficacy of GABAA α5 antagonist S44819 in patients with ischaemic stroke: a multicentre, double-blind, randomised, placebo-controlled trial.Lancet Neurol. 2020 Mar;19(3):226-233. doi: 10.1016/S1474-4422(20)30004-1. Lancet Neurol. 2020. PMID: 32085836 Clinical Trial.
-
A double-blind placebo-controlled clinical evaluation of MultiStem for the treatment of ischemic stroke.Int J Stroke. 2014 Apr;9(3):381-6. doi: 10.1111/ijs.12065. Epub 2013 May 22. Int J Stroke. 2014. PMID: 23692637 Clinical Trial.
-
Safety and efficacy of bone marrow mononuclear cell therapy for ischemic stroke recovery: a systematic review and meta-analysis of randomized controlled trials.Neurol Sci. 2024 May;45(5):1885-1896. doi: 10.1007/s10072-023-07274-x. Epub 2024 Jan 3. Neurol Sci. 2024. PMID: 38172413 Free PMC article. Review.
-
A review and meta-analysis of stem cell therapies in stroke patients: effectiveness and safety evaluation.Neurol Sci. 2024 Jan;45(1):65-74. doi: 10.1007/s10072-023-07032-z. Epub 2023 Sep 21. Neurol Sci. 2024. PMID: 37733251 Free PMC article. Review.
Cited by
-
Brain repair mechanisms after cell therapy for stroke.Brain. 2024 Oct 3;147(10):3286-3305. doi: 10.1093/brain/awae204. Brain. 2024. PMID: 38916992 Free PMC article. Review.
-
Revolutionizing Stroke Recovery: Unveiling the Promise of Stem Cell Therapy.Drug Des Devel Ther. 2024 Mar 29;18:991-1006. doi: 10.2147/DDDT.S460998. eCollection 2024. Drug Des Devel Ther. 2024. PMID: 38567255 Free PMC article. Review.
References
-
- Jauch EC, Saver JL, Adams HP Jr, et al. ; American Heart Association Stroke Council; Council on Cardiovascular Nursing; Council on Peripheral Vascular Disease; Council on Clinical Cardiology . Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(3):870-947. doi:10.1161/STR.0b013e318284056a - DOI - PubMed
-
- Turc G, Bhogal P, Fischer U, et al. . European Stroke Organisation (ESO)—European Society for Minimally Invasive Neurological Therapy (ESMINT) guidelines on mechanical thrombectomy in acute ischaemic stroke endorsed by Stroke Alliance for Europe (SAFE). Eur Stroke J. 2019;4(1):6-12. doi:10.1177/2396987319832140 - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical

