Research Progress on Structure-Activity Relationship of 1,8-Naphthalimide DNA Chimeras Against Tumor
- PMID: 38225189
- PMCID: PMC10793192
- DOI: 10.1177/15330338231225861
Research Progress on Structure-Activity Relationship of 1,8-Naphthalimide DNA Chimeras Against Tumor
Abstract
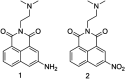
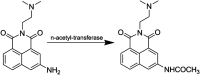
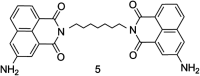
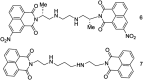
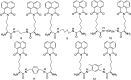
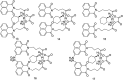
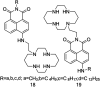
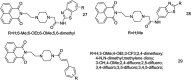
The development of 1,8-naphthalimide derivatives as cell probes, DNA targeting agents, and anti-tumor drugs is one of the research hotspots in the field of medicine. Naphthalimide compounds are a kind of DNA embedder, which can change the topological structure of DNA by embedding in the middle of DNA base pairs, and then affect the recognition and action of topoisomerase on DNA. Aminofide and mitonafide are the first 2 drugs to undergo clinical trials. They have good DNA insertion ability, can embed DNA double-stranded structure, and induce topoisomerase II to cut part of pBR322DNA, but not yet entered the market due to their toxicity. In this paper, the design and structure-activity relationship of mononaphthalimide and bisaphthalimide compounds were studied, and the relationship between the structure of naphthalimide and anti-tumor activity was analyzed and discussed. It was found that a variety of structural modifications were significant in improving anti-tumor activity and reducing toxicity.
Keywords: 1; 8-naphthalimides; DNA intercalator; antitumor; structure-activity relationship.
Conflict of interest statement
Declaration of Conflicting InterestsThe authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
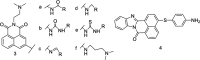
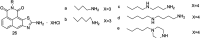
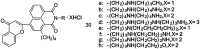
Similar articles
-
1,8-Naphthalimide: A Potent DNA Intercalator and Target for Cancer Therapy.Chem Rec. 2017 Oct;17(10):956-993. doi: 10.1002/tcr.201600134. Epub 2017 Apr 4. Chem Rec. 2017. PMID: 28375569 Review.
-
Naphthalimides and azonafides as promising anti-cancer agents.Curr Med Chem. 2009;16(10):1192-213. doi: 10.2174/092986709787846659. Curr Med Chem. 2009. PMID: 19355879 Review.
-
Anticancer Activity and Topoisomerase II Inhibition of Naphthalimides with ω-Hydroxylalkylamine Side-Chains of Different Lengths.Med Chem. 2019;15(5):550-560. doi: 10.2174/1573406414666180912105851. Med Chem. 2019. PMID: 30207241
-
Recent developments on 1,8-Naphthalimide moiety as potential target for anticancer agents.Bioorg Chem. 2022 Apr;121:105677. doi: 10.1016/j.bioorg.2022.105677. Epub 2022 Feb 12. Bioorg Chem. 2022. PMID: 35202852 Review.
-
Synthesis, electrochemistry, DNA binding and in vitro cytotoxic activity of tripodal ferrocenyl bis-naphthalimide derivatives.J Inorg Biochem. 2021 Jun;219:111425. doi: 10.1016/j.jinorgbio.2021.111425. Epub 2021 Mar 19. J Inorg Biochem. 2021. PMID: 33831713
Cited by
-
An Overview of Naphthylimide as Specific Scaffold for New Drug Discovery.Molecules. 2024 Sep 24;29(19):4529. doi: 10.3390/molecules29194529. Molecules. 2024. PMID: 39407459 Free PMC article. Review.
References
-
- Gopikrishna P, Meher N, Iyer PK. Functional 1,8-Naphthalimide AIE/AIEEgens: recent advances and prospects[J]. ACS Appl Mater Interfaces. 2018;10(15):12081-12111. - PubMed
-
- Jia T, Fu C, Huang C, et al. Highly sensitive naphthalimide-based fluorescence polarization probe for detecting cancer cells[J]. ACS Appl Mater Interfaces. 2015;7(18):10013-10021. - PubMed
-
- Johnson CA, Hudson GA, Hardebeck LK, et al. Effect of intercalator substituent and nucleotide sequence on the stability of DNA- and RNA-naphthalimidecomplexes[J]. Bioorg Med Chem. 2015;23(13):3586-3591. - PubMed
-
- Brana MF, Castellano JM, Roldan CM, et al. Synthesis and mode(s) of action of a new series of imide derivatives of 3-nitro-1,8 naphthalic acid[J]. Cancer ChemotherPharmacol. 1980;4(1):61-66. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

