The efficacy and safety of oral antibiotic treatment in patients with chronic low back pain and Modic changes: A systematic review and meta-analysis
- PMID: 38222804
- PMCID: PMC10782054
- DOI: 10.1002/jsp2.1281
The efficacy and safety of oral antibiotic treatment in patients with chronic low back pain and Modic changes: A systematic review and meta-analysis
Abstract
Background: This systematic review and meta-analysis aimed to summarize evidence regarding the effectiveness and safety of oral antibiotic intervention for chronic low back pain (CLBP) patients with/without type-1 Modic changes (MC1).
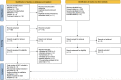
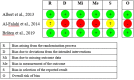
Methods: AMED, CINAHL, Cochrane Library, Embase, and Medline were searched from inception to March 3, 2023. Randomized controlled trials (RCTs) or non-RCTs that investigated the effectiveness or safety of oral antibiotics in treating CLBP patients were eligible for inclusion. Two independent reviewers screened abstracts, full-text articles, and extracted data. The methodological quality of each included article were evaluated by RoB2 and NIH quality assessment tools. The quality of evidence was appraised by GRADE. Meta-analyses were performed, where applicable. A subgroup analysis was conducted to evaluate the RCTs and case series separately, and to evaluate the effect of removing a low-quality RCT.
Results: Three RCTs and four case series were included. All Amoxicillin-clavulanate/Amoxicillin treatments lasted for approximately 3 months. Moderate- and low-quality evidence suggested that antibiotic was significantly better than placebo in improving disability and quality of life in CLBP patients with MC1 at 12-month follow-up, respectively. Low-quality evidence from meta-analyses of RCTs showed that oral antibiotic was significantly better than placebo in improving pain and disability in CLBP patients with MC1 immediately post-treatment. Very low-quality evidence from the case series suggested that oral Amoxicillin-clavulanate significantly improved LBP/leg pain, and LBP-related disability. Conversely, low-quality evidence found that oral Amoxicillin alone was not significantly better than placebo in improving global perceived health in patients with CLBP at the 12-month follow-up. Additionally, oral antibiotic users had significantly more adverse effects than placebo users.
Conclusions: Although oral antibiotics were statistically superior to placebo in reducing LBP-related disability in patients with CLBP and concomitant MC1, its clinical significance remains uncertain. Future large-scale high-quality RCTs are warranted to validate the effectiveness of antibiotics in individuals with CLBP.
Keywords: Modic change; low back; meta‐analysis; nucleus pulposus; pain; pain antibiotics; systematic review.
© 2023 The Authors. JOR Spine published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Conflict of interest statement
The authors have no financial or competing interests concerning this work to disclose.
Figures


Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Non-steroidal anti-inflammatory drugs for acute low back pain.Cochrane Database Syst Rev. 2020 Apr 16;4(4):CD013581. doi: 10.1002/14651858.CD013581. Cochrane Database Syst Rev. 2020. PMID: 32297973 Free PMC article.
-
Complementary and alternative therapies for back pain II.Evid Rep Technol Assess (Full Rep). 2010 Oct;(194):1-764. Evid Rep Technol Assess (Full Rep). 2010. PMID: 23126534 Free PMC article. Review.
-
Therapeutic ultrasound for chronic low back pain.Cochrane Database Syst Rev. 2020 Jul 5;7(7):CD009169. doi: 10.1002/14651858.CD009169.pub3. Cochrane Database Syst Rev. 2020. PMID: 32623724 Free PMC article.
-
Pharmacological treatments for low back pain in adults: an overview of Cochrane Reviews.Cochrane Database Syst Rev. 2023 Apr 4;4(4):CD013815. doi: 10.1002/14651858.CD013815.pub2. Cochrane Database Syst Rev. 2023. PMID: 37014979 Free PMC article. Review.
Cited by
-
Gut microbiome dysbiosis is associated with lumbar degenerative spondylolisthesis in symptomatic patients.JOR Spine. 2024 Oct 10;7(4):e70005. doi: 10.1002/jsp2.70005. eCollection 2024 Dec. JOR Spine. 2024. PMID: 39398942 Free PMC article.
-
Intradiscal administration of autologous platelet-rich plasma in patients with Modic type 1 associated low back pain: A prospective pilot study.JOR Spine. 2024 Mar 18;7(1):e1320. doi: 10.1002/jsp2.1320. eCollection 2024 Mar. JOR Spine. 2024. PMID: 38500785 Free PMC article.
-
Discovery of circulating blood biomarkers in patients with and without Modic changes of the lumbar spine: a preliminary analysis.Eur Spine J. 2024 Apr;33(4):1398-1406. doi: 10.1007/s00586-024-08192-y. Epub 2024 Mar 7. Eur Spine J. 2024. PMID: 38451373
References
-
- Hartvigsen J, Hancock MJ, Kongsted A, et al. What low back pain is and why we need to pay attention. Lancet. 2018;391:2356‐2367. - PubMed
-
- Foster NE, Anema JR, Cherkin D, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018;391:2368‐2383. - PubMed
-
- Chou R, Huffman LH. Nonpharmacologic therapies for acute and chronic low back pain: a review of the evidence for an American Pain Society/American College of Physicians clinical practice guideline. Ann Intern Med. 2007;147:492‐504. - PubMed
-
- Chaparro LE, Furlan AD, Deshpande A, Mailis‐Gagnon A, Atlas S, Turk DC. Opioids compared with placebo or other treatments for chronic low back pain: an update of the Cochrane review. Spine. 2014;39:556‐563. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

