Immunosurveillance encounters cancer metabolism
- PMID: 38216787
- PMCID: PMC10897436
- DOI: 10.1038/s44319-023-00038-w
Immunosurveillance encounters cancer metabolism
Abstract
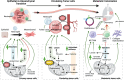
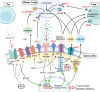
Tumor cells reprogram nutrient acquisition and metabolic pathways to meet their energetic, biosynthetic, and redox demands. Similarly, metabolic processes in immune cells support host immunity against cancer and determine differentiation and fate of leukocytes. Thus, metabolic deregulation and imbalance in immune cells within the tumor microenvironment have been reported to drive immune evasion and to compromise therapeutic outcomes. Interestingly, emerging evidence indicates that anti-tumor immunity could modulate tumor heterogeneity, aggressiveness, and metabolic reprogramming, suggesting that immunosurveillance can instruct cancer progression in multiple dimensions. This review summarizes our current understanding of how metabolic crosstalk within tumors affects immunogenicity of tumor cells and promotes cancer progression. Furthermore, we explain how defects in the metabolic cascade can contribute to developing dysfunctional immune responses against cancers and discuss the contribution of immunosurveillance to these defects as a feedback mechanism. Finally, we highlight ongoing clinical trials and new therapeutic strategies targeting cellular metabolism in cancer.
Keywords: Cancer Evolution; Immunoediting; Immunometabolism.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
New Immunometabolic Strategy Based on Cell Type-Specific Metabolic Reprogramming in the Tumor Immune Microenvironment.Cells. 2022 Feb 22;11(5):768. doi: 10.3390/cells11050768. Cells. 2022. PMID: 35269390 Free PMC article. Review.
-
Immunoediting instructs tumor metabolic reprogramming to support immune evasion.Cell Metab. 2023 Jan 3;35(1):118-133.e7. doi: 10.1016/j.cmet.2022.12.003. Cell Metab. 2023. PMID: 36599297 Free PMC article.
-
NAD-Biosynthetic and Consuming Enzymes as Central Players of Metabolic Regulation of Innate and Adaptive Immune Responses in Cancer.Front Immunol. 2019 Jul 25;10:1720. doi: 10.3389/fimmu.2019.01720. eCollection 2019. Front Immunol. 2019. PMID: 31402913 Free PMC article. Review.
-
Immunometabolic Checkpoints of Treg Dynamics: Adaptation to Microenvironmental Opportunities and Challenges.Front Immunol. 2019 Aug 27;10:1889. doi: 10.3389/fimmu.2019.01889. eCollection 2019. Front Immunol. 2019. PMID: 31507585 Free PMC article. Review.
-
The cancer metabolic reprogramming and immune response.Mol Cancer. 2021 Feb 5;20(1):28. doi: 10.1186/s12943-021-01316-8. Mol Cancer. 2021. PMID: 33546704 Free PMC article. Review.
Cited by
-
Role of Exosomes in Cancer and Aptamer-Modified Exosomes as a Promising Platform for Cancer Targeted Therapy.Biol Proced Online. 2024 May 27;26(1):15. doi: 10.1186/s12575-024-00245-2. Biol Proced Online. 2024. PMID: 38802766 Free PMC article. Review.
-
Lipids in the tumor microenvironment: immune modulation and metastasis.Front Oncol. 2024 Sep 26;14:1435480. doi: 10.3389/fonc.2024.1435480. eCollection 2024. Front Oncol. 2024. PMID: 39391242 Free PMC article. Review.
-
Function and mechanism of exosomes derived from different cells as communication mediators in colorectal cancer metastasis.iScience. 2024 Feb 27;27(4):109350. doi: 10.1016/j.isci.2024.109350. eCollection 2024 Apr 19. iScience. 2024. PMID: 38500820 Free PMC article. Review.
-
Cold and hot tumors: from molecular mechanisms to targeted therapy.Signal Transduct Target Ther. 2024 Oct 18;9(1):274. doi: 10.1038/s41392-024-01979-x. Signal Transduct Target Ther. 2024. PMID: 39420203 Free PMC article. Review.
References
-
- Akalay I, Janji B, Hasmim M, Noman MZ, Andre F, De Cremoux P, Bertheau P, Badoual C, Vielh P, Larsen AK, et al. Epithelial-to-mesenchymal transition and autophagy induction in breast carcinoma promote escape from T-cell-mediated lysis. Cancer Res. 2013;73:2418–2427. - PubMed
-
- Allard B, Turcotte M, Spring K, Pommey S, Royal I, Stagg J. Anti-CD73 therapy impairs tumor angiogenesis. Int J Cancer. 2014;134:1466–1473. - PubMed
-
- Andresen L, Hansen KA, Jensen H, Pedersen SF, Stougaard P, Hansen HR, Jurlander J, Skov S. Propionic acid secreted from propionibacteria induces NKG2D ligand expression on human-activated T lymphocytes and cancer cells. J Immunol. 2009;183:897–906. - PubMed
Publication types
MeSH terms
Grants and funding
- 31003A_182470/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (SNF)
- IZLCZO_206083/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung (SNF)
- 802773/EC | ERC | HORIZON EUROPE European Research Council (ERC)
- 111-2314-B-016-004/MOST | Institute for Information Industry, Ministry of Science and Technology, Taiwan (III)
- MND-MAB-D-112095/National Defense Medical Center (NDMC)
LinkOut - more resources
Full Text Sources

