Targeting cancer and immune cell metabolism with the complex I inhibitors metformin and IACS-010759
- PMID: 38214418
- PMCID: PMC11223609
- DOI: 10.1002/1878-0261.13583
Targeting cancer and immune cell metabolism with the complex I inhibitors metformin and IACS-010759
Abstract
Metformin and IACS-010759 are two distinct antimetabolic agents. Metformin, an established antidiabetic drug, mildly inhibits mitochondrial complex I, while IACS-010759 is a new potent mitochondrial complex I inhibitor. Mitochondria is pivotal in the energy metabolism of cells by providing adenosine triphosphate through oxidative phosphorylation (OXPHOS). Hence, mitochondrial metabolism and OXPHOS become a vulnerability when targeted in cancer cells. Both drugs have promising antitumoral effects in diverse cancers, supported by preclinical in vitro and in vivo studies. We present evidence of their direct impact on cancer cells and their immunomodulatory effects. In clinical studies, while observational epidemiologic studies on metformin were encouraging, actual trial results were not as expected. However, IACS-01075 exhibited major adverse effects, thereby causing a metabolic shift to glycolysis and elevated lactic acid concentrations. Therefore, the future outlook for these two drugs depends on preventive clinical trials for metformin and investigations into the plausible toxic effects on normal cells for IACS-01075.
Keywords: IACS‐010759; cancer; clinical trial; immunometabolism; metformin; microenvironment.
© 2024 The Authors. Molecular Oncology published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
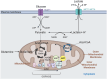
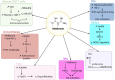
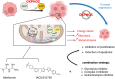
Similar articles
-
Why Metformin Should Not Be Used as an Oxidative Phosphorylation Inhibitor in Cancer Patients.Chemotherapy. 2023;68(4):185-189. doi: 10.1159/000531606. Epub 2023 Jun 21. Chemotherapy. 2023. PMID: 37343530 Review.
-
Mechanism-Specific Pharmacodynamics of a Novel Complex-I Inhibitor Quantified by Imaging Reversal of Consumptive Hypoxia with [18F]FAZA PET In Vivo.Cells. 2019 Nov 21;8(12):1487. doi: 10.3390/cells8121487. Cells. 2019. PMID: 31766580 Free PMC article.
-
Tumor-targeted nano-assemblies for energy-blocking cocktail therapy in cancer.Acta Biomater. 2024 Aug;184:368-382. doi: 10.1016/j.actbio.2024.06.023. Epub 2024 Jun 20. Acta Biomater. 2024. PMID: 38908417
-
Drugging OXPHOS Dependency in Cancer.Cancer Discov. 2019 Aug;9(8):OF10. doi: 10.1158/2159-8290.CD-ND2019-005. Epub 2019 Jun 11. Cancer Discov. 2019. PMID: 31186236
-
Immune-mediated anti-tumor effects of metformin; targeting metabolic reprogramming of T cells as a new possible mechanism for anti-cancer effects of metformin.Biochem Pharmacol. 2020 Apr;174:113787. doi: 10.1016/j.bcp.2019.113787. Epub 2019 Dec 27. Biochem Pharmacol. 2020. PMID: 31884044 Review.
Cited by
-
Fatty acid synthase (FASN) is a tumor-cell-intrinsic metabolic checkpoint restricting T-cell immunity.Cell Death Discov. 2024 Sep 30;10(1):417. doi: 10.1038/s41420-024-02184-z. Cell Death Discov. 2024. PMID: 39349429 Free PMC article.
-
The Involvement of Ascorbic Acid in Cancer Treatment.Molecules. 2024 May 13;29(10):2295. doi: 10.3390/molecules29102295. Molecules. 2024. PMID: 38792156 Free PMC article. Review.
-
Anti-Inflammatory Potential of the Anti-Diabetic Drug Metformin in the Prevention of Inflammatory Complications and Infectious Diseases Including COVID-19: A Narrative Review.Int J Mol Sci. 2024 May 10;25(10):5190. doi: 10.3390/ijms25105190. Int J Mol Sci. 2024. PMID: 38791227 Free PMC article. Review.
-
Dynamics of Mitochondrial Proteome and Acetylome in Glioblastoma Cells with Contrasting Metabolic Phenotypes.Int J Mol Sci. 2024 Mar 19;25(6):3450. doi: 10.3390/ijms25063450. Int J Mol Sci. 2024. PMID: 38542426 Free PMC article.
References
-
- Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

