Human adenovirus oncolytic properties and the inhibitory role of E4 orf4 and E4 orf6/7 on endogenously activated NF-κB
- PMID: 38205184
- PMCID: PMC10776911
- DOI: 10.1016/j.bbrep.2023.101616
Human adenovirus oncolytic properties and the inhibitory role of E4 orf4 and E4 orf6/7 on endogenously activated NF-κB
Abstract
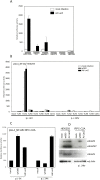
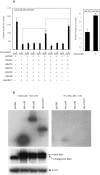
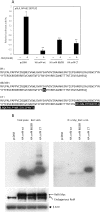
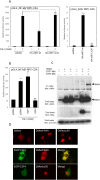
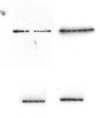
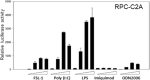
Human adenovirus is a promising tool for cancer therapy as an oncolytic virus. To predict which region of the oncolytic adenovirus E4 gene could be deleted, we investigated the relationship between the E4 proteins and NF-κB. Here, we report that TLR2-dependent NF-κB activation in Ad5-infected cells was significantly inhibited 24 h post-infection. Among the six E4 proteins, E4 orf4 and E4 orf6/7 exhibited notable suppressive effects on NF-κB activation. However, only E4 orf4 was co-immunoprecipitated with the RelA protein, also known as p65. It appears likely that E4 orf6/7 represses NF-κB activation via E2F-dependent pathways. Our results suggest that both E4 orf4 and E4 orf6/7 are novel inhibitors of NF-κB activation. The inhibition of endogenous NF-κB activation by E4 proteins during the late phase of infection also appears to elucidate the previously reported suppression of E1A expression in the late phase of infection. These redundant suppressive effects of E4 orf4 and E4 orf6/7 on NF-κB suggest that these proteins may play a major role in the anticancer properties of oncolytic adenovirus.
Keywords: Adenovirus; E4 orf4; E4 orf6/7; NF-κB; TLR2.
© 2023 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Adenovirus E4 open reading frame 4-induced dephosphorylation inhibits E1A activation of the E2 promoter and E2F-1-mediated transactivation independently of the retinoblastoma tumor suppressor protein.Virology. 1999 Apr 10;256(2):313-21. doi: 10.1006/viro.1999.9663. Virology. 1999. PMID: 10191196
-
Recombinant adenoviral vectors can induce expression of p73 via the E4-orf6/7 protein.J Virol. 2006 Jun;80(11):5349-60. doi: 10.1128/JVI.02016-05. J Virol. 2006. PMID: 16699015 Free PMC article.
-
Adenovirus E4 open reading frame 4 protein autoregulates E4 transcription by inhibiting E1A transactivation of the E4 promoter.J Virol. 1996 Jun;70(6):3844-51. doi: 10.1128/JVI.70.6.3844-3851.1996. J Virol. 1996. PMID: 8648720 Free PMC article.
-
Oncolytic adenovirus-induced autophagy: tumor-suppressive effect and molecular basis.Acta Med Okayama. 2013;67(6):333-42. doi: 10.18926/AMO/52006. Acta Med Okayama. 2013. PMID: 24356717 Review.
-
Regulation of mRNA production by the adenoviral E1B 55-kDa and E4 Orf6 proteins.Curr Top Microbiol Immunol. 2003;272:287-330. doi: 10.1007/978-3-662-05597-7_10. Curr Top Microbiol Immunol. 2003. PMID: 12747554 Review.
References
-
- Boivin D., Morrison M.R., Marcellus R.C., Querido E., Branton P.E. Analysis of synthesis, stability, phosphorylation, and interacting polypeptides of the 34-kilodalton product of open reading frame 6 of the early region 4 protein of human adenovirus type 5. J. Virol. 1999;73:1245–1253. doi: 10.1128/JVI.73.2.1245-1253.1999. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

