Combination Therapy with a TLR7 Agonist and a BRD4 Inhibitor Suppresses Tumor Growth via Enhanced Immunomodulation
- PMID: 38203835
- PMCID: PMC10779224
- DOI: 10.3390/ijms25010663
Combination Therapy with a TLR7 Agonist and a BRD4 Inhibitor Suppresses Tumor Growth via Enhanced Immunomodulation
Abstract
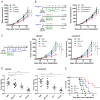
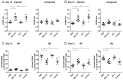
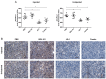
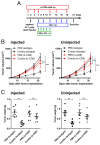
JQ-1 is a typical BRD4 inhibitor with the ability to directly fight tumor cells and evoke antitumor immunity via reducing the expression of PD-L1. However, problems arise with the development of JQ-1 in clinical trials, such as marked lymphoid and hematopoietic toxicity, leading to the investigation of combination therapy. SZU-101 is a TLR7 agonist designed and synthesized by our group with potent immunostimulatory activity. Therefore, we hypothesized that combination therapy of SZU-101 and JQ-1 would target innate immunity and adaptive immunity simultaneously, to achieve a better antitumor efficacy than monotherapy. In this study, the repressive effects of the combination administration on tumor growth and metastasis were demonstrated in both murine breast cancer and melanoma models. In 4T1 tumor-bearing mice, i.t. treatment with SZU-101 in combination with i.p. treatment with JQ-1 suppressed the growth of tumors at both injected and uninjected sites. Combination therapy increased M1/M2 ratio in TAMs, decreased PD-L1 expression and promoted the recruitment of activated CD8+ T cells in the TME. In summary, the improved therapeutic efficacy of the novel combination therapy appears to be feasible for the treatment of a diversity of cancers.
Keywords: BRD4; TAM; TLR7; antitumor; immunity.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
A chemical conjugation of JQ-1 and a TLR7 agonist induces tumoricidal effects in a murine model of melanoma via enhanced immunomodulation.Int J Cancer. 2021 Jan 15;148(2):437-447. doi: 10.1002/ijc.33222. Epub 2020 Sep 21. Int J Cancer. 2021. PMID: 32683685
-
PD-L1/TLR7 dual-targeting nanobody-drug conjugate mediates potent tumor regression via elevating tumor immunogenicity in a host-expressed PD-L1 bias-dependent way.J Immunother Cancer. 2022 Oct;10(10):e004590. doi: 10.1136/jitc-2022-004590. J Immunother Cancer. 2022. PMID: 36253000 Free PMC article.
-
Conjugate of ibrutinib with a TLR7 agonist suppresses melanoma progression and enhances antitumor immunity.Int J Biol Sci. 2022 Jan 1;18(1):166-179. doi: 10.7150/ijbs.64094. eCollection 2022. Int J Biol Sci. 2022. PMID: 34975325 Free PMC article.
-
Combination of Sunitinib and PD-L1 Blockade Enhances Anticancer Efficacy of TLR7/8 Agonist-Based Nanovaccine.Mol Pharm. 2019 Mar 4;16(3):1200-1210. doi: 10.1021/acs.molpharmaceut.8b01165. Epub 2019 Jan 25. Mol Pharm. 2019. PMID: 30620878
-
Toll-like receptor 7/8 agonist R848 alters the immune tumor microenvironment and enhances SBRT-induced antitumor efficacy in murine models of pancreatic cancer.J Immunother Cancer. 2022 Jul;10(7):e004784. doi: 10.1136/jitc-2022-004784. J Immunother Cancer. 2022. PMID: 35851308 Free PMC article.
References
-
- Hosoya T., Sato-Kaneko F., Ahmadi A., Yao S., Lao F., Kitaura K., Matsutani T., Carson D.A., Hayashi T. Induction of oligoclonal CD8 T cell responses against pulmonary metastatic cancer by a phospholipid-conjugated TLR7 agonist. Proc. Natl. Acad. Sci. USA. 2018;115:E6836–E6844. doi: 10.1073/pnas.1803281115. - DOI - PMC - PubMed
-
- Michaelis K.A., Norgard M.A., Zhu X., Levasseur P.R., Sivagnanam S., Liudahl S.M., Burfeind K.G., Olson B., Pelz K.R., Angeles Ramos D.M., et al. The TLR7/8 agonist R848 remodels tumor and host responses to promote survival in pancreatic cancer. Nat. Commun. 2019;10:4682. doi: 10.1038/s41467-019-12657-w. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

